
The current picture of pharmaceutical regulatory industry is somewhat like this: globalization in emerging markets, increasing concerns of public health by health authorities and stringent regulatory guidelines to adhere to. For companies to avail the uninterrupted supply of its medical products and devices in the market, swift pre and post marketing approvals are required. Consequent to which most of the companies now choose a dedicated regulatory service provider that delivers best practices for the most intricate tasks instead of taking risks of approval failures.
With regulatory requirements becoming complex day by day, the trend of regulatory outsourcing has taken over the pharma regulatory market. In fact as per a report shared by Transparency Market Research, global regulatory affairs outsourcing market was valued at USD 1.56 billion in 2013 and is expected to grow at a CAGR of 14.6% from 2014 to 2020, to reach an estimated value of USD 4.49 billion in 2020.
If we look at other reasons as to why is there such a huge shift by pharma companies towards outsourcing partners, there are a lot of advantages for them than just getting the work done. A professional regulatory outsourcing partner can offer resource flexibility, centralized process development lifecycle, additional capacity, first-time-right tasks, and most importantly significant reduction and savings in overall cost of compliance. Companies tend to save approximately 50-80% on the cost of compliance if regulatory services are outsourced to a promising partner.
The dedicated and skilled regulatory outsourcing providers not only facilitate the companies to get timely approvals for the products, but also help the health authorities to save time on reviewing the documents and submissions. The first-time-right services help saving significantly on resources, effort and time.
Going Beyond Traditional Outsourcing Services
Even though companies have started opting for regulatory outsourcing partners, the trends in services dynamically change every now and then. The year 2013-2014 witnessed a commendable growth of more than 40% in just the regulatory publishing and writing services out of the entire regulatory affairs outsourcing market. However earlier the main focus persisted on the functional tasks such as dossier management and publishing & submissions, now we see new doors unlocked for companies for new regulatory undertakings like regulatory intelligence, CMC and labeling and regulatory data management.
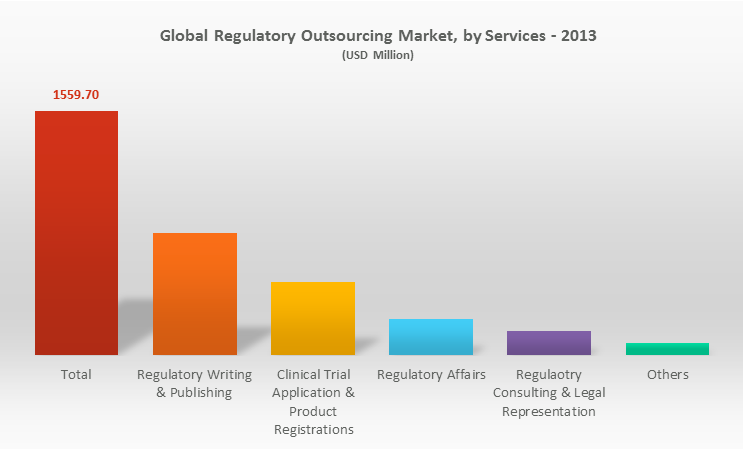
Source: KOL Opinions, Company Annual Report, Expert Interviews, Investing Publications, Press Releases and TMR Reports
On the global front, the largest regulatory outsourcing market with more than 30% of the share is accounted in North America. However the globalization of medical products has set new anticipations for emerging markets of Asia Pacific countries like Japan, Thailand, India, and China. As per the reports, these countries will turn out to be a major hub of regulatory sourcing services with a growth rate expected to be more than 15% during the period of 2014-2020 mainly due to accessibility of extensive resources, skilled manpower and smooth market gateways for developing economies. Professionals in regulatory space suggest the service providers to measure the outcomes as per customer requirements in terms of deliverables or service level metrics. Such parameters help both the partners to harmonize well, being at different geographical locations.
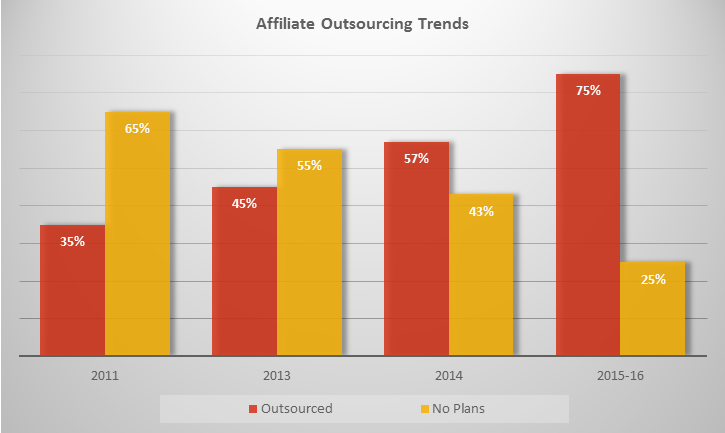
Conclusion
The shifts in regulatory outsourcing trends simply signify the ongoing transitions in the industry driven by guidelines and regulations put forward by health authorities. The struggle to meet these guidelines led to improved and strategic regulatory services for the companies. In the coming future, the trends in outsourcing are expected to provide extensive resources and intelligent plans for companies expanding their businesses, especially in emerging markets.









