Market Intelligence Service: Data-Driven Strategies for Medical Device Overview
Are you confident your medical device market strategy is up to date? Without proper market intelligence, you might miss key opportunities, lag behind competitors, or face regulatory challenges. Our Market Intelligence Service provides the insights needed to drive innovation, stay competitive, and ensure smooth market entry.
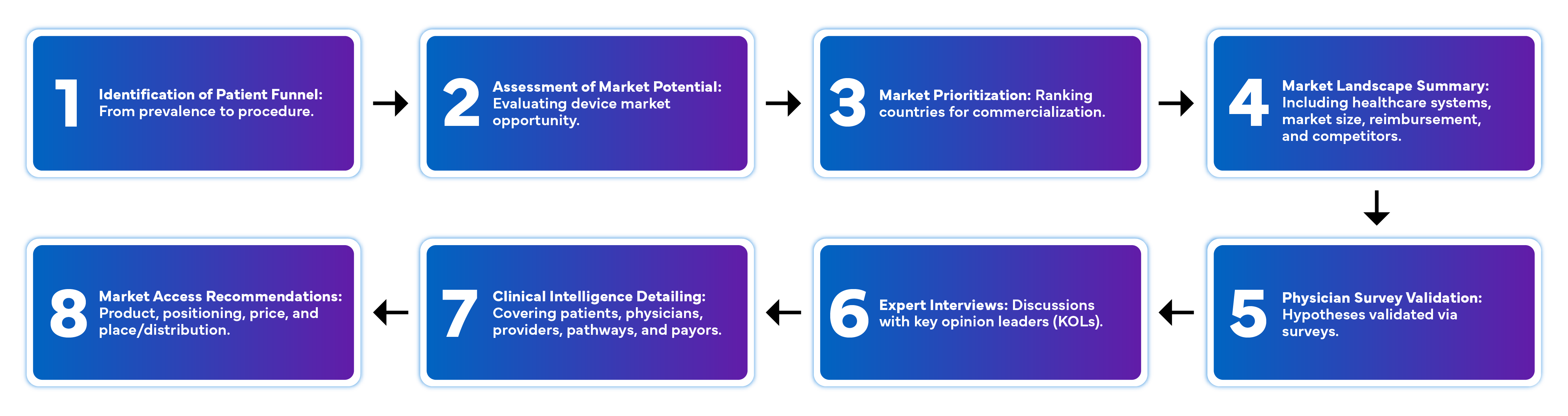
Advantages of Market Intelligence
Discover how comprehensive medical device market research can streamline your product development and market entry.:
- Stay informed about the latest medical device industry trends and how they can benefit your product development and innovation.
- Support on for refinement for your market approach strategy with insights into trends in the medical device industry.
- Stay up-to-date with the latest regulatory changes to ensure compliance and avoid potential market access issues.
Did You Know?
- 60%
face unexpected regulatory challenges from insufficient market Insights - 40%
fail to assess competition accurately, resulting in strategic missteps - 35%
of medical device companies overestimate demand, leading to overproduction and inventory costs due to poor market intelligence. - 30%
experience failures from geographics misalignment, entering markets that don't align with strategic goals
These statistics highlight the critical importance of comprehensive market research for medical devices for successful market entry and sustained growth
Freyr’s Market Intelligence Services Offered