Overview
In the highly regulated food and food supplements industry, ensuring raw materials meet global Food Safety Regulations requirements is crucial for product quality, safety, and market access. Compliance with raw material specifications for Supplements helps manufacturers mitigate risks, maintain product consistency, and achieve Regulatory approval across different regions.
Why Raw Material Specification Compliance Matters
Regulatory Approval
Adhering to regulations tailored to specific countries such as the FDA, EFSA, FSANZ, TGA, FSSAI, SFDA, MFDS, MHLW, NHC
Product Safety
Ensuring that raw materials are free from contaminants, allergens, and other harmful substances.
Quality Assurance
Maintaining ingredient potency, purity, and composition consistency.
Market Access
Avoiding product recalls and import rejections due to non-compliance.
Major Regulations that affect the Raw Material Compliance Globally
- Applicable - USA
- Compliance Scope: Provides internationally recognized standards for the quality, identity, and purity of food ingredients regulatory requirements.
- Applicable - EU Member States
- Compliance Scope: Lays down specifications for food additives listed in Annexes II and III of Regulation (EC) No 1333/2008, detailing purity criteria and usage conditions.
- Applicable: Globally
- Compliance Scope: Provides safety evaluations and establishes acceptable daily intakes (ADIs) for food additives regulatory approval, contaminants, naturally occurring toxicants, and residues of veterinary drugs in food.
Raw materials used in Food and Food supplements include
Botanical Extracts
Vitamins & Minerals
Amino Acids
Probiotics & Enzymes
Flavors & Sweeteners
Preservatives & Additives
Proteins
Key Regulatory Requirements

Identification & Purity Standards
Identification & Purity Standards
Verify ingredient identity and purity as per regional guidelines.

Contaminant Limits
Contaminant Limits
Heavy metals, pesticides, microbiological, and residual solvents compliance.

Allergen Declarations
Allergen Declarations
Clear labeling and ingredient traceability.

Country-Specific Compliance
Country-Specific Compliance
Meeting the requirements of different regulatory bodies such as FDA (USA), EFSA (EU), NMPA (China), and ANVISA (Brazil).

Specification Documentation
Specification Documentation
Comprehensive Certificates of Analysis (CoA), Technical Data Sheets (TDS), and Material Safety Data Sheets (MSDS).
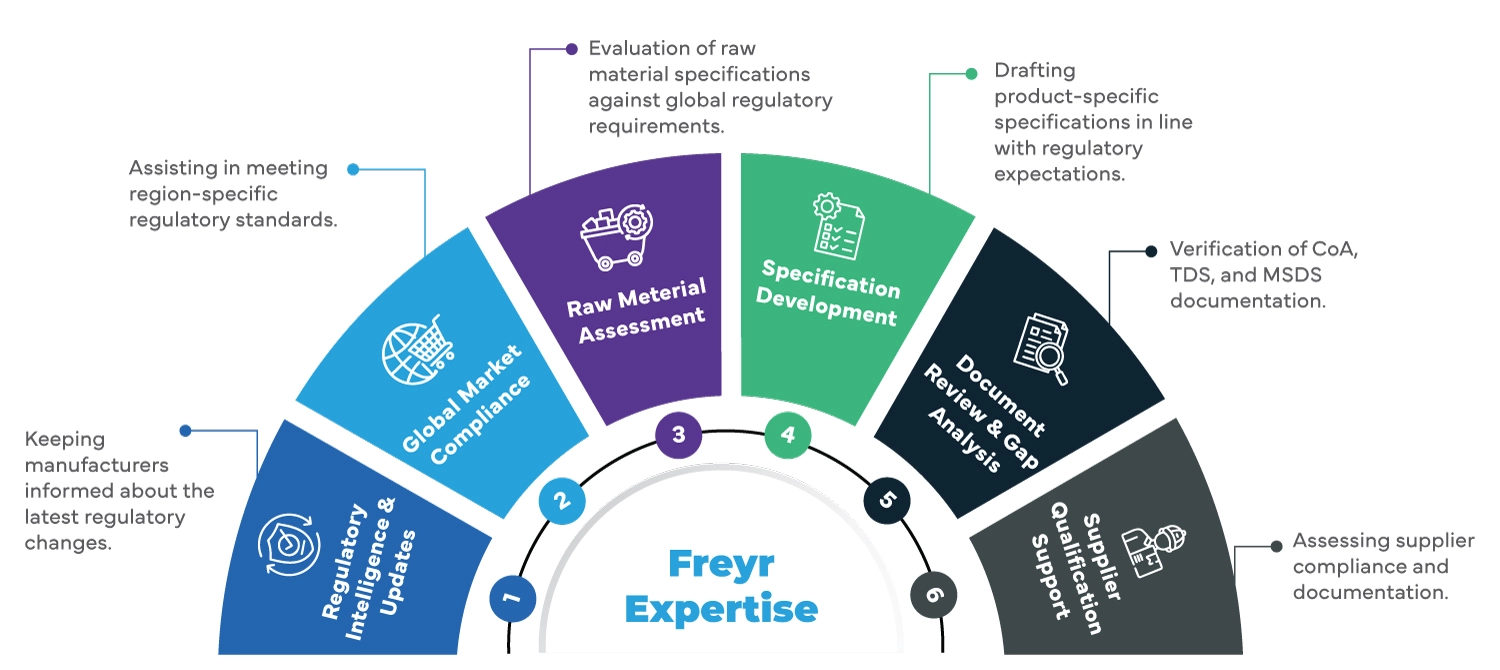
Freyr's Expertise in Raw Material Specification Compliance
Freyr provides end-to-end support for manufacturers and suppliers to achieve Regulatory compliance for Food raw materials used in food and dietary supplements. Our services include:
Why Choose Freyr?

End-to-End Regulatory Support
End-to-End Regulatory Support
From strategy to submissions, ensuring complete compliance with global raw material regulation.

Market Knowledge
Market Knowledge
A team of experts with profound knowledge of the Food and Food Supplements market entry landscape.

Expertise Across Categories
Expertise Across Categories
Proficient in handling Food and food Supplements, tobacco, pet food, animal feed, and other food-related product categories.

Tailored and Cost-Effective Solutions
Tailored and Cost-Effective Solutions
Customizable and cost-effective solutions tailored to client needs.

Regulatory Compliance Excellence
Regulatory Compliance Excellence
Proven track record in regulatory compliance services.
Ensure your raw materials meet global Regulatory standards with Freyr's comprehensive compliance solutions. Contact us today to learn more about how we can support your Regulatory journey.
























