Medical and Scientific Communication - Overview
Are you bringing a new brand or product onto the market? Are you focused on the post-approval promotional requirements of a product? Does your company have an effective medical communication strategy, means, and channels to disseminate and consume scientific information?
From our decades of extensive life sciences industry experience, we adeptly transform the complexity of medical concepts and scientific data to impactful and engaging narratives that epitomize the convergence of our Creativity, Therapeutic area knowledge, Scientific accuracy, and Regulatory requirements proficiency.
Empower your Medical Affairs communication for diverse target audiences - HCPs, Patients, KOLs, MSLs, etc.- with our team of specialized experts in Medical Copywriting, Medical and scientific writing, Creative Scientific Artwork, Content Marketing, and Regulatory Consulting.
Navigate the complex and dynamic landscape of your Medicinal Products and Medical Devices' Advertisement and Promotional Labeling Regulatory compliance with Freyr, your strategic partner!
Freyr’s Medical and Scientific Communication
Medical and Scientific Communication
CME Assets
Continuing Medical Education (CME)
Conference Assets
Conference Abstracts, Posters, Presentations
Scientific Publications
Scientific Articles, Journal Publications, Publication Extenders
Product Literature
Product brochures, Fact sheets, REMS materials, Sales aids
KOL / MSL Engagement
KOL engagement and MSL slide decks
Multilingual Content Management
Content transcreation and adaptation for local markets
Creative Scientific Design Studio
Bioimages, Scientific illustrations, Infographics, Brochures, Flyers, REMS materials, MoAs, IFUs, eLearning modules, Awareness Videos, Standees, Banners & Posters for Events/ Conferences/ Symposia.
MLR Compliance
Medical, Legal and Regulatory (MLR) compliance reviews
Reference Validation
Claims Substantiation, Fact Checks, and Reference Validation
Global Regulatory Expertise
Regulatory compliance with country-specific guidelines for 120+ countries FDA, EMA, MHRA, TGA, HSA, PMDA, ANVISA, Health Canada, Medicines Australia, Medicines New Zealand and others.
Ethical Code Compliance
Compliance with industry body ethical codes worldwide for 120+ countries, such as PAAB, ABPI, PAGB, ASA, PhRMA, IFPMA, and PSA, but not limited to.
Labelling Submission
Advertisement and Promotional labeling Submission to the Health Authority, such as OPDP/APLB Form 2253 to the US FDA in manual and eCTD formats.
Digital Promotional Assets
Content distribution is done through various digital channels such as websites, emailers, and social media.
Lifecycle Management
MLR Coordination and Life Cycle Maintenance of assets
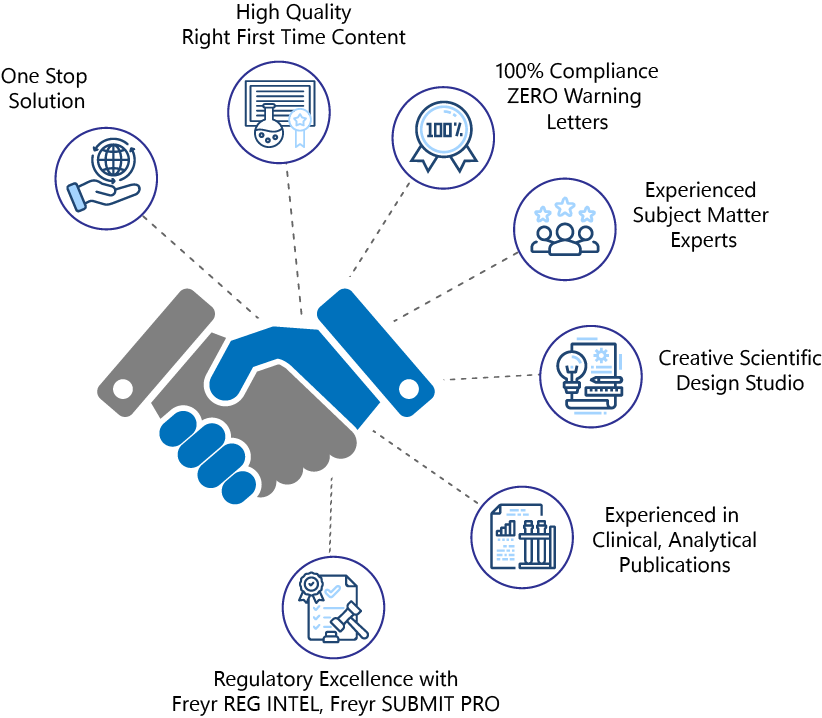
Why choose Freyr as your medical communications partner?
- One-Stop Shop for all your Promotional and Medical Publication needs - Content Creation, MLR Compliance, HA Submission, Digital Content Marketing.
- High-quality ‘Right First Time’ content tailored to resonate with various target audiences.
- Our MLR Review outcomes ensure 100% Compliance, ZERO Warning Letters, and No notice of Violation.
- Niche blend of qualified Subject Matter Experts in ‘Ad Promo’, Medical and Scientific Publications, and Regulatory Insights (Medics, PhDs, and Pharmacists).
- Cutting-edge creative design studio for Life Sciences Marketing and Medical Communication.
- Extensive medical publishing experience in positioning clinical outcomes, comparative analysis presentation, and industry-oriented publications in high-impact journals.
- Regulatory Excellence powered by Freyr Reg Intel and Freyr SUBMIT Pro for up-to-date Regulatory requirements and hassle-free ‘Ad Promo’ Submission to HA.

 Medical & Scientific Communication
Medical & Scientific Communication







