Performance Evaluation for Medical Devices - Overview
The Software as a Medical Device (SaMD) market is booming globally given factors such as rising demand for remote health services and the upcoming innovation in the digital health space. In Europe, the SaMD market is anticipated to grow at approximately 27.1% during the time period of 2021 - 2027.
SaMD includes an In-vitro Diagnostic (IVD) medical device. It is crucial to emphasize that the EU regulations do not employ the term "Software as a Medical Device (SaMD)." Instead, they utilize the term "Medical Device Software," abbreviated as MDSW.
In the European Union (EU), MDSW is governed by the Medical Device Regulation (MDR) 2017/745 and the In-Vitro Diagnostics Regulation (IVDR) 2017/746. These regulations provide a framework for ensuring the safety and performance of medical devices, including SaMDs, within the EU market.
How does software qualify as a medical device?
To determine if your device is considered SaMD by the EU, you need to assess whether the software is intended to be used for one (01) or more medical purposes without being part of a hardware medical device. If your software operates on its own to fulfill a medical purpose, it could be qualified as SaMD.
However, if it is intended to drive a hardware medical device or is an integral part of one, it would not be considered standalone software and thus not SaMD. Always ensure that your product complies with the latest EU regulations and guidelines for medical devices.
How to register your SaMD in the EU?
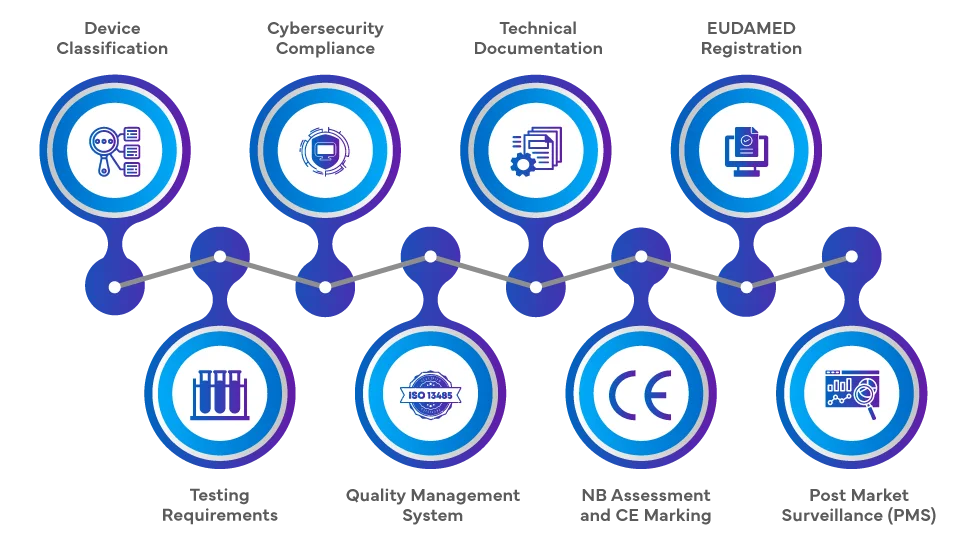
The Regulatory pathway for introducing SaMD into the EU market under the EU MDR 2017/745 and EU IVDR 2017/746 involves a comprehensive series of steps. Manufacturers must classify the software product based on its purpose and risk level, followed by a conformity assessment, which may involve a Notified Body (NB) for higher-risk classes. Technical documentation, clinical evaluation, and adherence to Quality Management Systems (QMSs) are crucial. Upon successful assessment, the CE marking is affixed, and registration in the European Database on Medical Devices (EUDAMED) is necessary. Continuous Post-market Surveillance (PMS), vigilance, and adherence to cybersecurity requirements complete the Regulatory journey.
Frequently Asked Questions (FAQs)
An EU MDR/IVDR certificate is a document issued by a designated NB as a result of a conformity assessment under the European Union's Medical Device Regulation (EU MDR 2017/745) or In-Vitro Diagnostics Regulation (IVDR) 2017/746. This certificate verifies that a medical device meets the requirements set out in the EU MDR/IVDR, which includes safety, performance, and quality standards. The certificate is necessary for medical devices / In-vitro diagnostic devices to be legally marketed within the EU.
In the EU, medical devices are classified into four (04) risk classes: Class I, Class IIa, Class IIb, and Class III. Class I represents devices with the lowest risk, and Class III includes devices with the highest risk. The classification considers factors such as the invasiveness of the device, the duration of use, the part of the body affected, and the potential risks associated with the device's technical design and manufacture.
In the same manner, SaMD is also classified according to the risk it poses to patients and users, ranging from low risk (Class I) to high risk (Class III).
The term "Software as a Medical Device (SaMD)" is applied when the software is designed for one (01) or more medical purposes, independently performing these functions without being an integral part of a hardware medical device. On the other hand, "Software in a Medical Device (SiMD)" is utilized when software is integrated into medical equipment, known as embedded software or firmware.
It is crucial to emphasize that the EU regulations do not employ the term "Software as a Medical Device." Instead, they utilize the term "Medical Device Software," abbreviated as MDSW.
"Medical Device Software (MDSW)" refers to software intended for use, either on its own or in combination, for a purpose outlined in the definition of a "Medical Device" as per the EU MDR 2017/745 and EU IVDR 2017/746.
The time it takes to get an SaMD approved in the EU can vary significantly based on several factors. The key determinants include the device's classification, complexity, conformity assessment route, the involvement of an NB, and the efficiency of the Regulatory submission process.
SaMD Registration in the other markets (EU/Australia/Korea) for Medical Devices
- Comprehensive EU MDR/IVDR Regulatory strategy for SaMDs.
- Regulatory and market intelligence support.
- Product classification and registration services for SaMDs.
- Regulatory support for SaMD product development documents.
- Consultation services on SaMD clinical evaluation studies.
- Post-approval change management.
- EAR/UKRP/CH-Rep services.

- Successful submissions for various classes of SaMDs.
- Dedicated and expert personnel to provide Regulatory support.
- On-time submission of deliverables.
- Local affiliate access to meet Health Authority (HA) challenges and language-specific requirements.
- In-country or Legal Representative (LR) support, with a cost-effective model.
- Regulatory resource management/staff augmentation services.









