Medical Device Importers in Canada Overview
A medical device importer in Canada is an individual or entity in Canada, responsible for bringing medical devices into the country for sale. This means the importer assumes the responsibility of importing medical devices from overseas into the Canadian market. The importer ensures that the medical devices they bring in comply with relevant Canadian regulations and are properly registered with Health Canada before being sold within the country.
Why the Medical Device Importer in Canada?
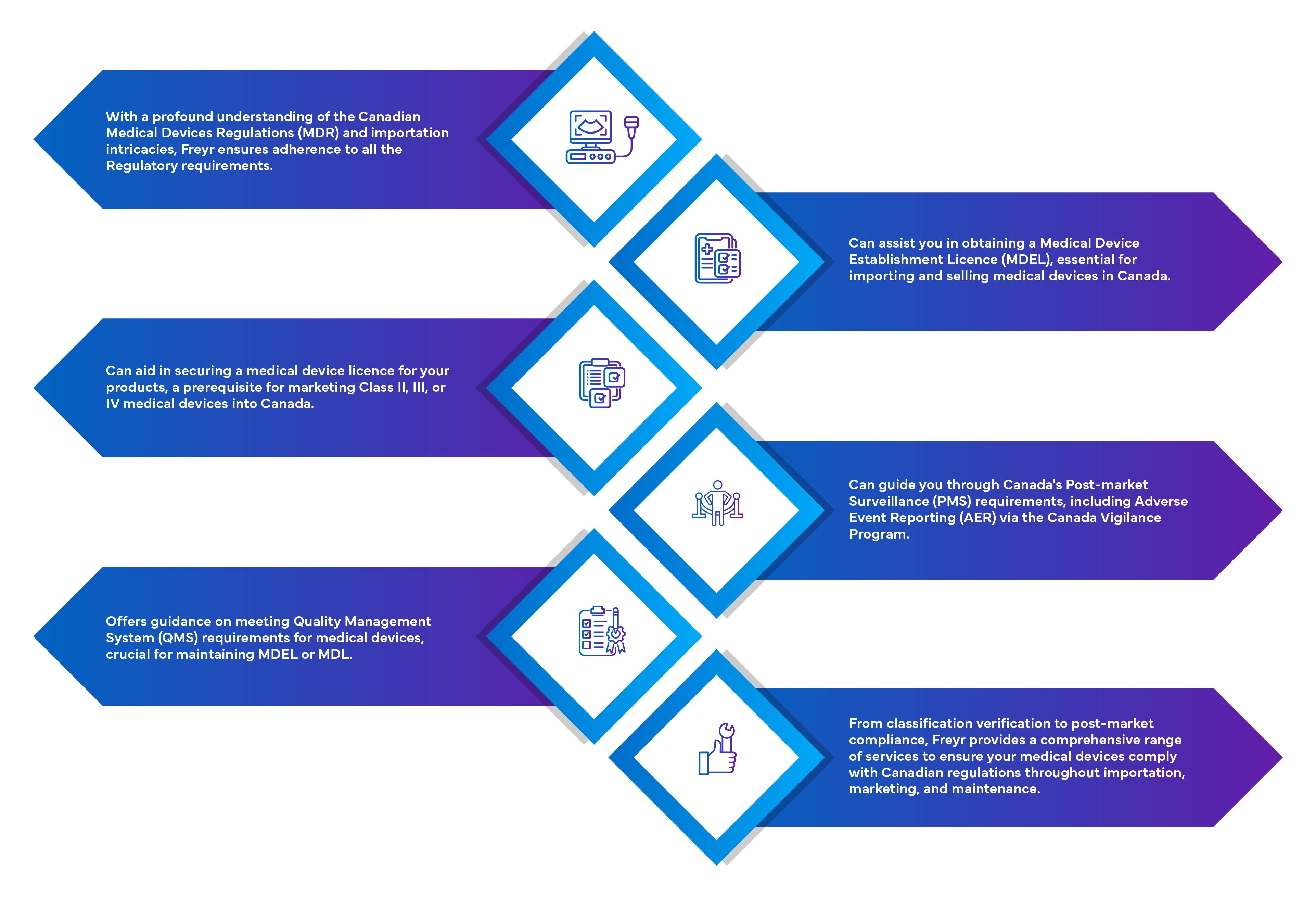
Importers must obtain a Medical Device Establishment License (MDEL) to import medical devices for sale in Canada. It is a Regulatory requirement to have an importer for the foreign manufacturer of Class I, II, III & IV devices to market the device in Canada.
What are the Requirements for a Medical Device Importer in Canada?
Importers must obtain a Medical Device Establishment License (MDEL) to import medical devices for sale in Canada.
Importers must maintain a physical address located within Canada and adhere to Health Canada's Regulatory requirements, including handling complaints, reporting incidents, and maintaining distribution records.
Streamline your Medical Device Importation process with our expert support. Contact Freyr's specialists today to ensure your medical device meets all the Regulatory requirements and achieves timely market access in Canada.
What Advantages do Class I Foreign Medical Device Manufacturers have in Designating an Importer with an MDEL?
Medical device manufacturers outside of Canada who only sell Class I medical devices in Canada do not require any additional license to market their devices, if their importer has an MDEL.
Freyr Medical Device Importer Competencies in Canada:
![]()
MDSAP, Canada.![]()
Distributor identification/Qualification of distributor for compliance with Health Canada requirements.![]()
Support for MDEL/MDL amendments.![]()
Medical Device Establishment Licence (MDEL) application.![]()
Labeling services as per Health Canada labeling requirements for medical devices.![]()
Reviewing of the labeling documents.
Frequently Asked Questions (FAQs)
You can verify the validity of an importer, foreign distributor, or foreign manufacturer's MDEL by accessing Health Canada's medical devices establishment licence listing database.
Certain entities or individuals are exempt from specific importation requirements if they fall under the following categories:
- Retailers or companies selling directly to end-users for personal use.
- Health care facilities.
- Manufacturers of Class II, III, or IV devices selling specific types of medical devices or custom-made devices.
- Persons importing or selling veterinary medical devices.
- Dispensers or individuals making or adapting medical devices for specific patient needs as directed by a professional.
Medical Device Regulatory Consulting – Proven Expertise
Why Freyr?