Medical Device Literature Search Protocol and Review – Overview
In the intricate realm of medical devices and In Vitro Diagnostics (IVD), the significance of a well-structured medical device literature search protocol and review transcends mere research exploration. It emerges as a cornerstone for achieving compliance, with stringent safety and performance requirements set forth by regulations like the European Union Medical Device Regulation (EU MDR) 2017/745 and European Union In Vitro Diagnostic Regulation (EU IVDR) 2017/746.
EU MDR Literature Review
Literature review plays a crucial role in the lifecycle management of a medical device. A systematic EU MDR literature search strategy is the backbone for the success of clinical evaluation, performance evaluation, as well as even post-market surveillance and post-market clinical/performance follow-up reports. Anchored by a systematic literature search strategy, this review is not just a phase, but also a guiding beacon toward informed decision-making.
The Power of a Robust Scientific Literature Synthesis Team
Manufacturers navigating the intricate realm of medical devices and IVDs require more than a routine literature review. A robust scientific literature synthesis team thorough with therapeutic area expertise is the compass that guides them through the labyrinth of Regulatory requirements, ensuring that compliance is not just met but, in fact, exceeded.
EU MDR Literature Search Protocol
The following phases constitute the EU MDR literature search protocol process:
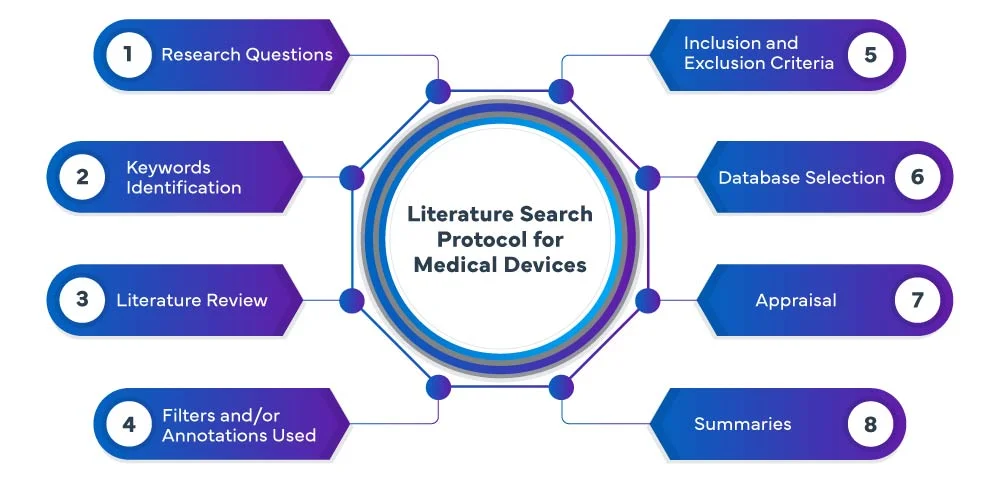
At Freyr, we conduct a systematic and exhaustive medical device literature search protocol and review across various databases, including PubMed, Embase, and Cochrane, to identify relevant studies and publications related to your medical device. Our team utilizes advanced search strategies to ensure that we capture all relevant evidence. We meticulously analyze and summarize the findings, providing you with a comprehensive review that serves as the foundation for your device’s development or evaluation process.
We recognize that every medical device is unique, and each product category requires tailored solutions. We work closely with you to understand your requirements and provide you with customized services that meet your objectives effectively.
Medical Device Literature Search Protocol and Review
- Identifying, searching, analyzing, and putting together the appropriate scientific literature.
- Strategizing the search strings and inclusion/exclusion of the criteria.
- Identifying the apt database for literature search report, as per the requirements.
- Collating the literature data.
- Screenings of the relevant literature.
- Integrating PMS data (if applicable).
- Documenting and reporting.
- Creation of the Clinical Evaluation Report (CER), as per EU MDR 2017/745 regulations.
- Creating a Clinical Evaluation Plan (CEP) for your organization.
- Conducting gap assessment of existing CEPs.

- Assured compliance with recent applicable regulations.
- A team of qualified clinical experts.
- Team scalability.
- Solutions tailored to your requirements.
- Cross-functional inputs from medical device experts to comply with requirements.
- Full-scope service from compliance, review, and planning.









