Health Canada Medical Device Registration Overview
Canada, with some of the stringent guidelines, has one of the best Regulatory systems in the world for medical devices. In Canada, all Medical Devices are regulated by Health Canada, Health Products and Food Branch, Therapeutic Products Directorate, Medical Devices Bureau. Health Canada reviews Medical Devices to assess their safety, effectiveness, and quality before being authorized for sale in Canada as per the Canada Medical Device Regulation SOR/98-282, implemented in 1998. Freyr has been an active partner of medical device companies to help them comply with the Health Canada medical device registration guidelines.
Regulatory Authority: Health Canada
Regulation: Medical Devices Regulations (SOR/98-282)
Authorized Representative: Not Required
QMS Requirement: ISO 13485:2016 compliance as Medical Device Single Audit Program (MDSAP)
Assessment of Technical Data: Health Canada
Validity of License: Unlimited
Labeling Requirements: Part 21 of MDR (SOR/98-282)
Submission Format: Paper
Language: English & French
Health Canada Medical Device Classification
The Health Canada Medical Device classification system is borrowed significantly from the European Union’s Council Directive 93/42/EEC. Many of the rules and interpretations of terms are like, those proposed by the European Union. It does not necessarily hold true, however, that a Medical Device classified in one class according to the European Union’s classification system will be classified in the same class based on the Canada Medical Device classification system. Applicant must follow the rules set out in the Regulations to determine the appropriate classification for their device in Canada.
The following indicators of risk posed by a given device were used to create the Canadian classification rules: degree of invasiveness, duration of contact, body system affected, and local versus systemic effects.
| Device Class | Risk |
|---|---|
| I | Low |
| II | Low-Moderate |
| III | High-Moderate |
| IV | High |
Canada Authorized Representative
There is no requirement for the manufacturer to appoint an Authorized Representative in Canada. The distributor, however, shall comply with the Health Canada requirements for Good Distribution Practices (GDP).
Medical Device Registration-Canada
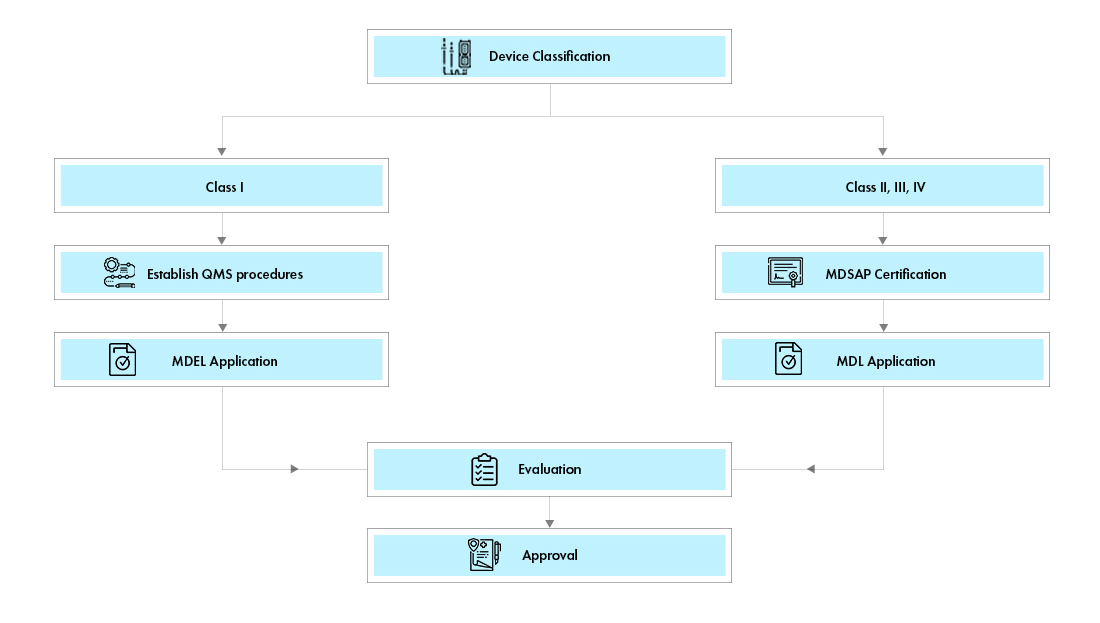
There are two pathways for medical device registration in Canada:
Medical Device Establishment License (MDEL): Class I devices can apply for Medical Device Establishment License Canada (MDEL) by preparing mandatory procedures and paying Health Canada fees.
Medical Device License (MDL): Class II, III, and IV devices shall apply for a Canadian Medical Device License (MDL) application. The document requirements for each of the device class vary.
Process flow
Post Approval Device Life Cycle Management
Freyr supports foreign manufacturers in end-to-end Medical Device lifecycle Management, including post approval activities, such as:
- Post approval change management - modifications to existing medical device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between Health Canada and the manufacturer
Summary
| Risk | Device Class | QMS Audit | Regulatory Pathway | Document Requirements | Health Canada Timelines |
|---|---|---|---|---|---|
| Low | I | NA | MDEL |
| NA |
| Low-Moderate | II | MDSAP certificate | MDL |
| 15 Days |
| High-Moderate | III | MDSAP certificate | MDL |
| 60 Days |
| High | IV | MDSAP certificate | MDL |
| 75 Days |
Freyr’s Medical Device Device Registration Services
Freyr Expertise
- Health Canada Medical Device Classification and grouping services
- Medical Device Registration, Canada
- Pre-Submission meetings with Health Canada
- MDSAP, Canada
- Distributor Identification/Qualification of distributor for compliance with Health Canada requirements
- Medical Device Establishment Licence Canada (MDEL)
- Medical Device Licensing in Canada (MDL)
- Post Approval Change Management
- Labeling services as per Health Canada labeling requirements for Medical Devices