Regulatory Document Formatting Services- Overview
In the complex landscape of Regulatory submissions, our regulatory document formatting services ensure that documents are accurately formatted and compliant with agency standards—a process known as Pharmaceutical Submission Formatting—can be a daunting challenge for pharmaceutical, biotechnology, and medical device companies. Inconsistent documentation can lead to delays, rejections, and increased costs.
At Freyr, we recognize that preparing and submitting a pharma regulatory document is a crucial phase in the product development lifecycle. Our Regulatory Document Formatting Services are designed to offer comprehensive support for all your Regulatory publishing needs, including complex eCTD Document Formatting, ensuring legible documentation and assistance with special characters, fonts, and styles.
Every company must have a standardized style sheet. This standardization ensures that documents generated by different team members are uniform in appearance, facilitating seamless integration when merging data from various sources. By adhering to a standardized style, discrepancies and overlaps in data presentation are avoided, resulting in a cohesive and professional final document.
Customization and Flexibility
Our experts cater to different customizations that clients may request as standard practice. We understand that different companies have unique style requirements for Regulatory Document Formatting, and we pride ourselves on our flexibility to accommodate these needs. Our adaptable approach ensures that we can personalize our Regulatory Document Formatting Services to fit your company's distinct style preferences, ensuring a seamless and professional final document.
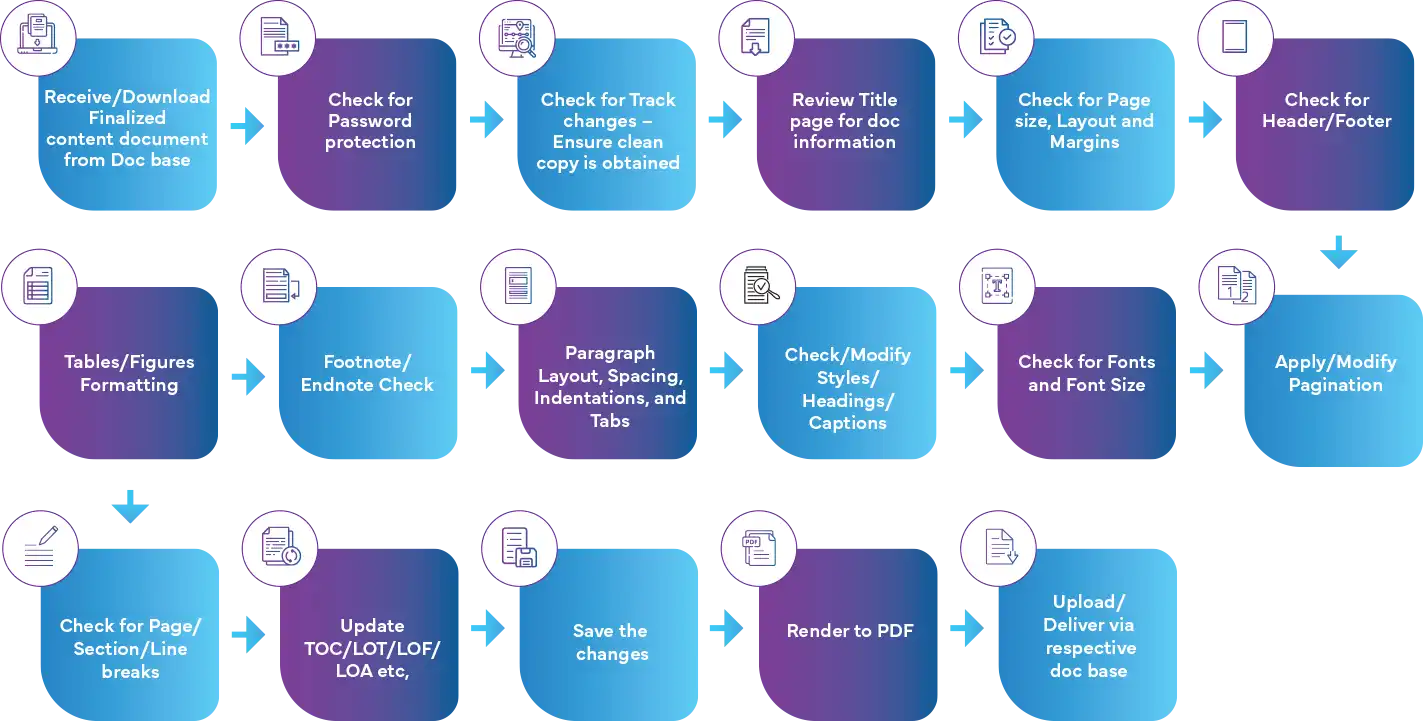
Tailored Templates for Regulatory Compliance
Freyr offers a range of customizable templates, from Model 1 to Model 5, specifically designed to incorporate data across various sections such as clinical, non-clinical, and CMC (Chemistry, Manufacturing, and Controls). These templates ensure that your documents are not only compliant with Regulatory standards but also tailored to your company's specific requirements.
Commitment to Quality
At Freyr, we are committed to providing our clients with high-quality Regulatory Document Formatting Services that streamline the Regulatory submission process. Our attention to detail and adherence to Regulatory standards ensure that your documents are submission-ready, helping you achieve faster approval times and market entry.
Regulatory Document Formatting Services
- We have a dedicated team for formatting various types of documents with precision.
- We develop customized templates to ensure standardization and consistency in document creation.
- Our style sheets ensure that document content meets client expectations.
- We use a meticulous, process-driven approach to eliminate discrepancies in developed documents.
- We offer ready-to-use templates for swift document development.
- We have a proven track record with over 50,000 pharma regulatory documents developed for various applications like IND, ANDA, and NDA.
- Our templates are accepted across different markets including the US, EU, HC, and GCC.
- We use ICH-recommended granular templates to ensure accurate data capture.
- Our detailed templates provide clear guidance on information to be filled in each section, enhancing clarity and accuracy.

- Freyr provides tailor-made approaches to meet diverse client expectations, ensuring personalized service.
- Our team has the ability to quickly adapt to last-minute changes, providing flexibility and responsiveness.
- We have expertise in formatting complex tables, figures, and appendices to meet client and Health Authority (HA) requirements.
- We can upscale or downscale resources based on the priority of activities, ensuring efficient resource management.
- Our comprehensive in-house style guides are perfect for clients seeking to harmonize content across different functions.
