
The Therapeutic Goods Administration (TGA) in Australia has stringent rules for registering new prescription medicines with novel active ingredients. Recently, the TGA has come up with a revised process to improve the efficacy of life-saving prescription drugs and make them available to patients faster. These changes are pertinent to applications that need to be supported by nonclinical, clinical, and/or bioequivalence data (category 1 and category 2). TGA released a document in August with details of the Regulatory requirements for the same.
Significant Features in the New Registration Process for Prescription Medicines
- The TGA has come up with a phase-wise registration process and has named the stages as milestones. Each of them follows a separate pathway.
- The new process aims to enhance the dossier quality. Dossiers are prepared based on a common technical document (CTD) format and follow all the Regulatory requirements.
- The pre-submission planning phase is important as applicants lodge details of the prospective application. This is done at least two and a quarter (02 ¼) months before the submission of the completed dossier. This stage is to help the TGA come up with the dates of the subsequent milestones and get the resources ready to manage the CTD.
- The next phase is submission. Once this is done, the TGA does not allow the applicant to offer any additional data or information after the submission date. It is mandatory to submit a complete dossier that is correct and of high quality.
- Under section 31 of the Therapeutic Goods Act 1989, the TGA requests additional information at the evaluation stage. After the initial evaluation, all the requests are collated and sent to the applicant.
To understand the milestones in the renewed process, please refer to the following flowcharts.
Milestone 1
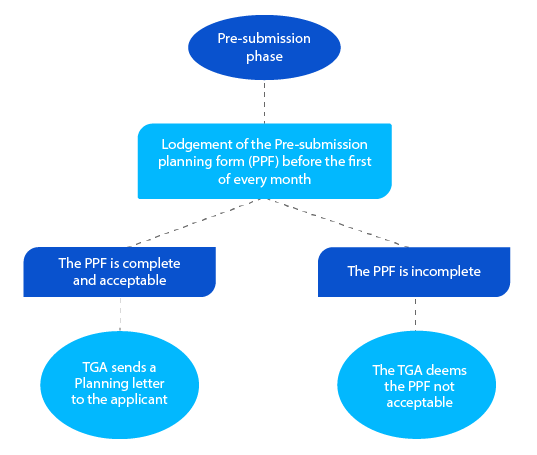
The Planning letter contains the expected date on which the TGA is expecting the lodgement of the dossier and other target milestone dates for the prescription drug application.
Milestone 2
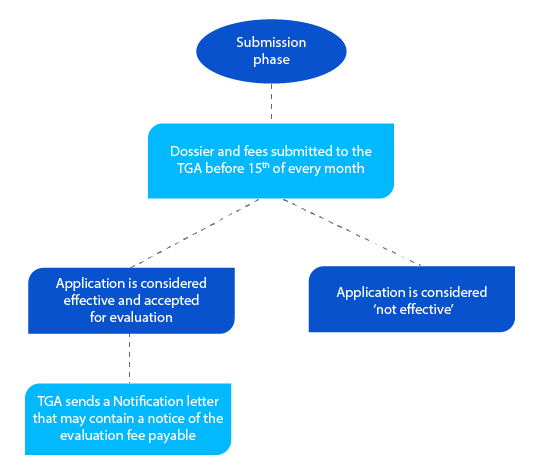
The dossier contains information that helps TGA evaluate the application, and if everything is acceptable, a Notification letter is sent before the end of the month in which the applicant submitted the dossier.
Milestone 3
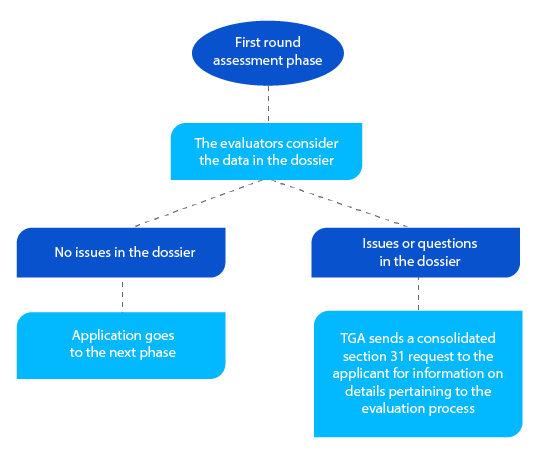
In addition to the consolidated section 31, request whenever applicable, the TGA sends copies of the assessment reports prepared by the quality, nonclinical, clinical, and Risk Management Plans (RMP) evaluators.
Milestone 4
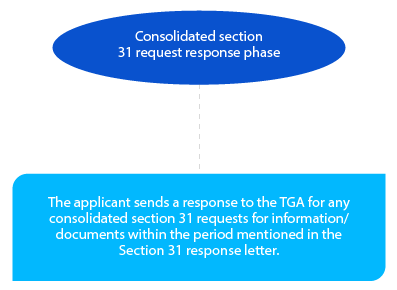
The applicant can nominate the section 31 response time. It can either be thirty (30) or sixty (60) days and should be highlighted in the PPF. This period needs to be confirmed by the TGA in the Planning Letter. The acceptable format of the response is CTD, and the same has to be sent in the form of hard and electronic copies.
Since this is the last chance for the applicant to provide any missing information to the TGA, it has to be done in a compliant manner.
Milestone 5
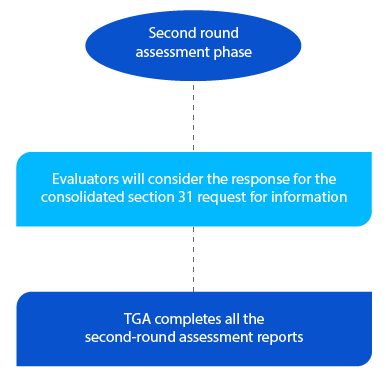
TGA gets two (02) months for new generic medicines applications for the second round of assessment reports and one (01) month for all other application types. The reports are sent to the respective applicants after the completion of the second assessment.
Milestone 6
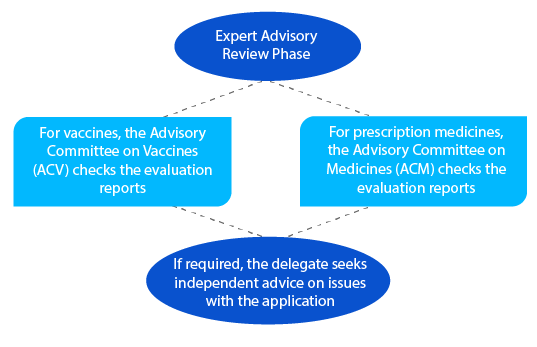
Post the advisory review, the TGA sends a notification with details of the advice that it receives from the ACV or the ACM.
Milestone 7
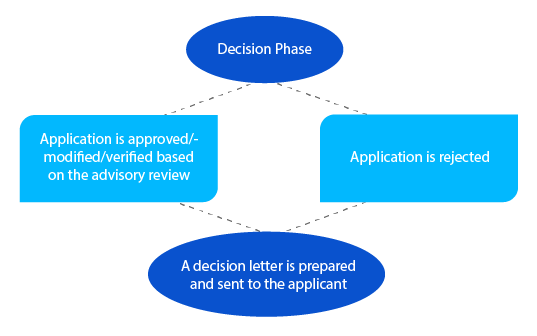
In case of pending issues, the delegate may liaise with the applicant in this phase before taking a decision. For applications under section 23 of the Therapeutic Goods Act of 1989 (newly assessed listed medicines in the Australian Register of Therapeutic Goods (ARTG)), a notification is sent to the applicant within twenty-eight (28) days of making the decision.
Milestone 8
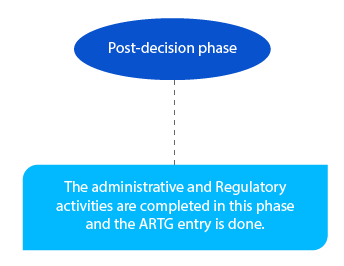
The outstanding payments are taken care of at this stage. And it has to be completed by the end of the month following the delegate’s decision.
With the TGA taking every precaution to ensure the safety, efficacy, and quality of the prescription drugs while reducing their time to market, the onus lies on the applicant to make the relevant submissions for a smooth registration process. Be it at any stage, collaborating with an end-to-end Regulatory services provider who is an expert in registering prescription drugs is the ideal situation. Contact an experienced Regulatory entity for a compliant pathway; stay updated and stay compliant.









