
As the world is moving towards digital transformation, the introduction of various tools is empowering many industries, including Pharmaceuticals and Life Sciences. In fact, the serialization and traceability of items can be improved even further as the world transitions to a digital economy. Currently, traditional approaches for achieving traceability in the Pharmaceutical Supply Chain are frequently centralized and sometimes lack transparency among supply chain participants. For this reason, Web3 tools, including Blockchain and Non-Fungible Tokens (NFTs) have several advantages, including speed, security, traceability, transparency, and accessibility of data provenance that would allow manufacturers to fight against counterfeit drugs. Because of its distinctiveness and digital signature, NFT is difficult to trade with other systems. Therefore, the NFT can act as the ideal digital replica of any physical product disseminated throughout a supply chain. Such integration within the traceability process might offer an impenetrable solution against fake medicines. Although there is a process, pharmaceutical manufacturers must integrate to achieve this digital transformation.
In Europe, the 2011/62/EU Directive signifies that all prescription drugs must have a unique identifier and, thus, has to be serialized to effectively fight against fake medicines. But what could be the actual steps to improve traceability and reduce counterfeit drug circulation in the market using the Web3 applications is discussed below.
Firstly, the integration of blockchain allows for making a decentralized system, which improves the traceability process and simplifies the integration among key players within the product distribution supply chain. With each step of the product distribution, users (such as manufacturers or hospitals) are issued private keys that allow them to perform fundamental functions of signing and retrieving data. Such keys can be variable in cryptography and used to encrypt or decrypt data, thus giving an extra layer of security and prevention of data breaches.
Although, a second item must be included in the equation to complete a successful blockchain-based traceability solution. An NFT acts as a token that provides the confirmation of product identification and assigns ownership to the user. Once the medicinal product is manufactured and is ready to move from the manufacturing site to the pharmaceutical factory, an NFT event must update the owner with the rightful details to pass the ownership token to the wholesale distributor. This also indicates that the manufacturer must modify the new owner’s public address of the NFT for the package. Once the wholesale distributor receives the package, a new event called ‘delivered’ is initiated within the blockchain, along with additional details about the product’s state. After the confirmation, the wholesaler initiates the token take over to the next user (the hospital or the pharmacy), which now acts as the last distributor, which must update the information about the received package in the system and guarantee that the product can be administered (or sold) to the patient. After the patient receives the product, the NFT is erased by transferring ownership to an unreachable address, preventing anybody else from ever changing the NFT data. A described supply chain can be seen in figure 1.
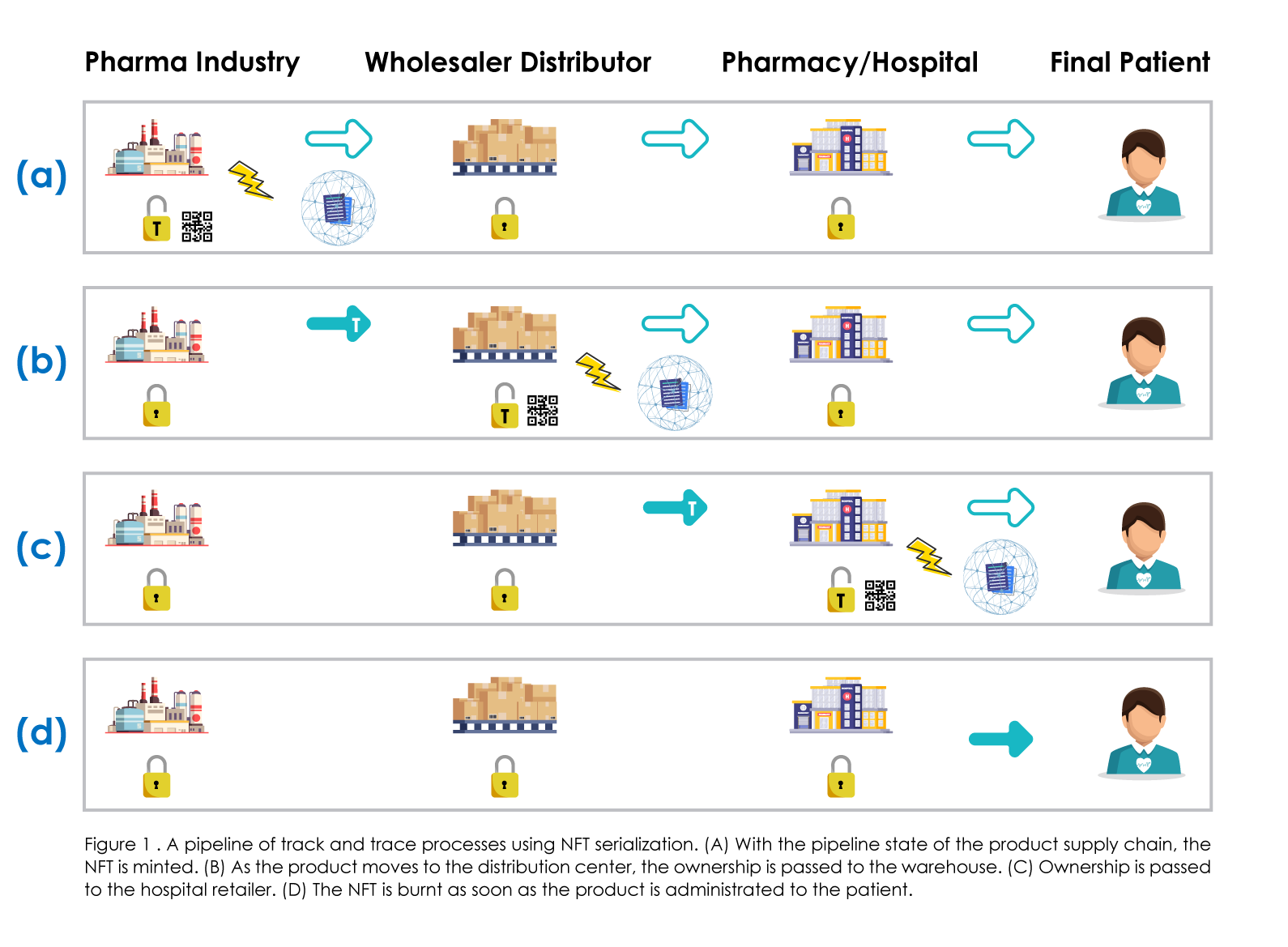
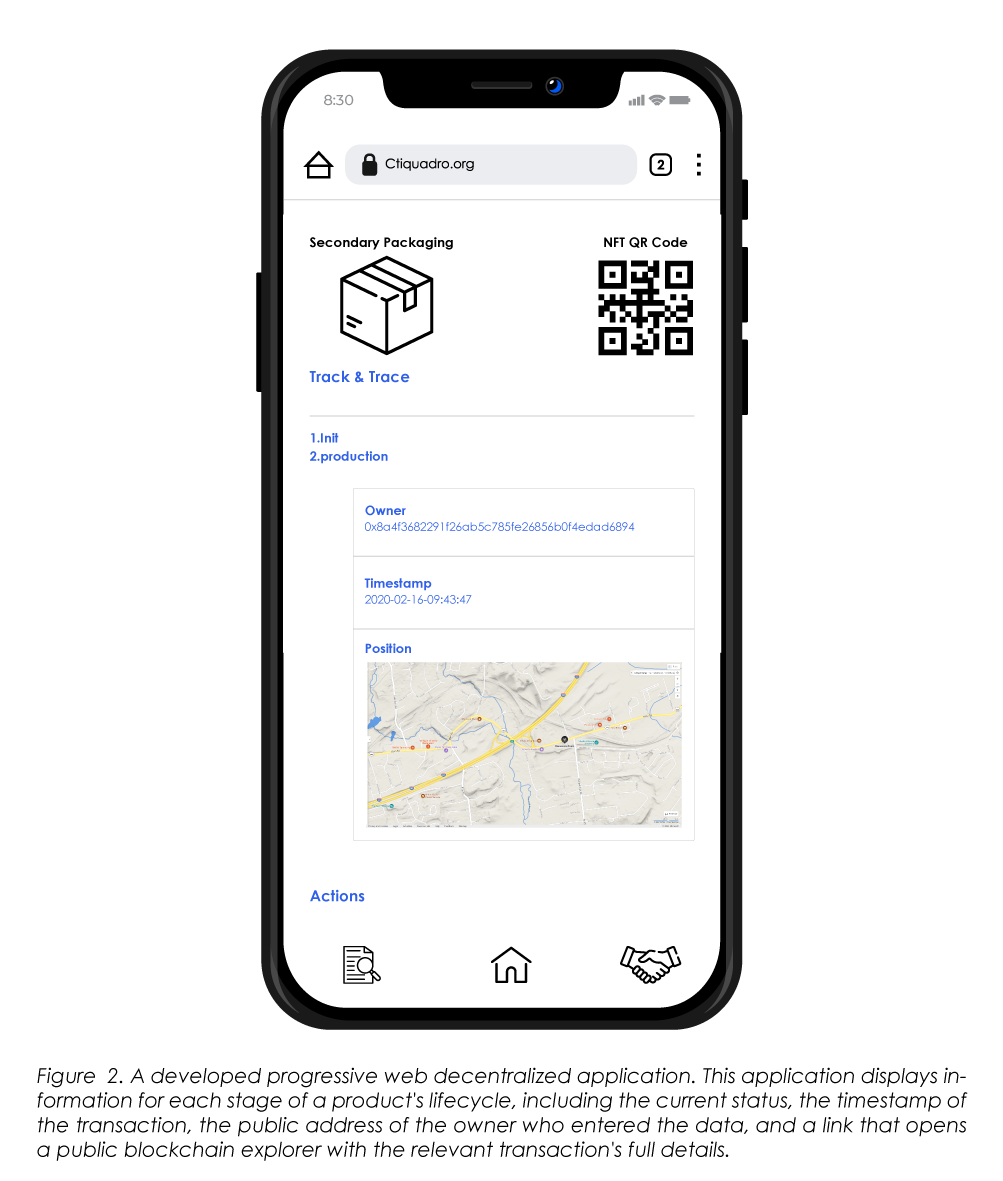
The traceability procedure that is discussed herein (Figure 1) gives the final customer an effective instrument for verifying the product's history and determining if it is in the right condition to be sold. The transparency of the blockchain throughout the entire production and distribution process enables all users to examine the status of each item by scanning the QR code with a Web3 application (Figure 2). This makes it possible to prevent and stop attempts at counterfeiting. Thus, the user who is receiving the package will be able to check the NFT's status and confirm the latest, even within the product distribution cycle, and whether the status of the movement was updated rightfully.
Web3 and all the tools it has to offer, though, will be more integrated in the coming years. What current options for many manufacturers or the supporting operational bodies have is the efficient and compliant Artwork Management System, which ensures valid tracking of the manufactured product. Although establishing an effective Artwork and Labeling strategy may sound like an overwhelming task, for this reason, Freyr provides expertise in the end-to-end Lifecycle Management of packaging Artwork services catering to a range of global Regulations. Our Regulatory experts guide pharmaceutical companies through every step of their product packaging journey.
To learn more about Freyr’s Artwork lifecycle coordination services, we are more than happy to have a quick call to understand what you might be seeking. Contact Freyr!









