
Medical devices play a vital role in improving patient health, but non-conformities in the devices can occur even with the best quality control systems in place. Non-conformities refer to instances where a product or a process fails to meet Regulatory requirements, quality standards, or the approved in-house procedures. Effective non-conformance management is important for ensuring patient safety, Regulatory compliance, and the reputation of medical device manufacturers.
This blog explores the importance of non-conformance management in medical devices, the process, and the best practices.
The Importance of Non-Conformance Management in Medical Devices
Effective non-conformance management can help identify potential issues early on, prevent harm to patients, and improve the overall quality of medical devices. It can also help manufacturers comply with Regulatory requirements, maintain customer satisfaction; and ultimately, improve the bottom line by reducing costs and manpower associated with product failures and recalls.
On the other hand, if non-conformities are not identified and addressed promptly, they can lead to device failure, Regulatory non-compliance and can have harmful effects on patient health. If a non-conforming product is released into the market, it can result in expensive recalls, legal repercussions, and damage to the reputation of the manufacturer.
The Process of Non-conformance Management in Medical Devices
The process of non-conformance management in medical devices involves several steps, including identification, investigation, Root Cause Analysis (RCA), impact assessment, and planning Corrective and Preventive Action(s) (CAPA). The process should be well-documented with non-conformance reports, and all stakeholders should be trained in the process to ensure its effectiveness.
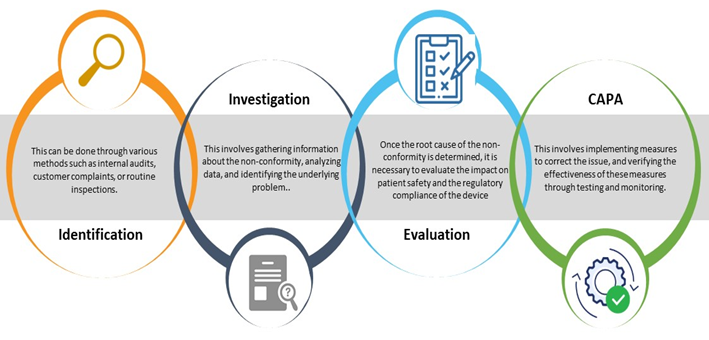
Five (05) Practices You Can Adopt for Effective Non-conformance Management in Medical Devices
Effective non-conformance management in medical devices requires a systematic and proactive approach to identifying and addressing non-conformities. The following are some of the best practices for effective non-conformance management in medical devices:
- A Robust Quality Management System (QMS)-: Establish and maintain an effective QMS that includes procedures for identifying and managing non-conformances.
Personnel Training: Ensure that all personnel are trained on non-conformance management procedures, including the process on how to identify, investigate, evaluate, carry out an impact assessment, and undertake CAPA.
- Record Maintenance: Maintain all records and document any information related to non-conformance tracking, RCA, impact assessment, and CAPA.
- Regular Internal Audits: Conduct internal audits regularly to assess the effectiveness of the non-conformance management process and identify areas of improvement.
Continuous Improvement: Foster a culture of continuous improvement by encouraging feedback from all stakeholders, including customers, suppliers, and employees.
It is crucial to implement effective non-conformance management to ensure patient safety, Regulatory compliance and protect the reputation of medical device manufacturers. You can achieve this by creating a robust QMS that can accurately identify and address non-conformities, ultimately preventing their occurrence and improving the overall quality of medical devices. Create a robust QMS for your medical device and ensure its compliance and patient safety with assistance from our Regulatory experts. Stay informed! Stay compliant!









