
It is tough for Pharma companies to be cognizant of all existing global regulatory requirements owing to the ever changing worldwide regulations and legislations. New procedures are always being developed and adopted by regulatory authorities worldwide due to the international harmonization process.
HISTORY OF REGULATORY INTELLIGENCE (RI)
RI typically is part of a company’s global regulatory affairs department and broadens the traditional regulatory affairs function beyond preparing and submitting applications to the FDA and the regulatory agencies of Europe and Asia.
The company’s leadership is kept informed about current regulations affecting the development, approval and maintenance of products, as well any changes to the regulations and/or regulatory landscape that may impact their efforts.
RI contributes to every pharmaceutical company’s bottom line by helping the regulatory affairs teams provide the highest quality submissions to agencies. Company must also understand issues that affect the review of new drug applications in each market in the world apart from regulatory guidance’s.
THE REGULATORY ENVIRONMENT
The constant shifts and changes in the regulatory environment requires all the concerned parties/authorities to be abreast of the current information from a variety of sources. Regulatory affairs professionals can tap industry practices, regulatory agency opinions, competitor information to develop successful regulatory strategies.
REGULATORY INTELLIGENCE (RI)
RI is the act of gathering and analysing regulatory information for impact or changes in laws, regulations, directives, guidance documents, etc. RI allows a regulatory professional to advise personnel, answer strategic regulatory questions and write or construct a global marketing application in addition determine requirements for conducting global clinical trials and manufacturing requirements.
RI offers insights on sourcing, filtering, analyzing and applying information to create valuable regulatory intelligence. Regulatory Intelligence follows a systematic process to streamline the functions within Regulatory Affairs.
HOW DOES REGULATORY INTELLIGENCE WORK FOR YOU?
Before applying for any clinical approvals, the Regulatory Affairs department comes up with a few questions to study the pros and cons of introducing a particular drug/product in different regions and other compliance needs. The research questions are driven by the business needs and linked to decisions and actions.
The Regulatory Intelligence process enables flexible research analysis. It extensively covers the complete product range that includes: Drugs, Devices, Biologicals, Veterinary Products, Consumer & OTC Products as well as Nutraceuticals.
The Regulatory information includes: New Guidelines, Guidelines Amendment,Pharmaceutical Development,Manufacturing, Quality, Clinical & Non Clinical, Stability & Storage, Validation,Packaging & Labeling, Pharmaceutical excipients, Impurities, Artwork & Promotional Material, Recall Alert,Safety & Pharmacovigilance, Warnings,Submission Formats and much more.
GLOBAL REGULATORY STRATEGY: SIGNIFICANCE
Change in the global landscape can affect the global regulatory strategy as more companies are conducting trials and filing marketing application worldwide. RI professionals benchmark regulatory developments across different countries, evaluate the adequacy of existing regulatory frameworks in view of latest technological developments or conduct regulatory analysis in the context of due diligence reports.
REGULATORY PRECEDENCE
A regulatory strategy can be developed through monitoring and gathering of RI which can result in:
• Reduced time to approval
• Maximization of target markets
• Decreased cost of product development based on current information
SOURCES OF REGULATORY INTELLIGENCE
Regulatory Precedence | Industry Practices | Regulatory Agency Opinions |Agency’s Websites | Guidance Documents | E-Mails from Regulatory Websites | Interactions with Agency Reviewers | Warning Letters | Colleagues & Consultants | FOI Requests| Competitor Information | Relevant Journals & Newsletters | Relevant Conferences | Advisory Meetings | Interactions with other Regulatory Professionals
PHARMACEUTICAL COMPANIES AND REGULATORY INTELLIGENCE
New business models including alternative information management platforms are being evaluated by leading pharmaceutical companies to reduce costs, shorten timelines and maintain quality and compliance. In the current regulatory climate, most of the Pharmaceutical companies are struggling to maintain R&D productivity.
Pharma companies are keen to explore new drug development models which can cut development costs, accelerate timelines and still maintain quality and compliance. They intend to realize these gains by slowing easing into new business models for data and regulatory management.
Innovative information management platforms that can manage the full scope of regulatory and clinical data operations with support across all geographies and regulatory agencies are being explored by Pharma companies. Information management platforms make research data more widely available by standardizing how it is collected formatted and distributed and offer unique perspectives to the investigative process.
Using analytics tools and techniques researchers can provide model outcomes, spot trends and ask the right questions which in turn will help the companies to standardize and optimize processes. Process reengineering efforts will involve more activity in areas of regulatory, Pharmacovigilance and clinical processes.
These in turn will be aided by integrated technology and analytics capabilities enabling global regulatory compliance. The swift turn to modernize their development operations is partly to do away with obsolete models of development in particular, in-house technology solutions that will in turn offset slowing growth rates to achieve bottom line results.
BUSINESS INTELLIGENCE AND PHARMA INDUSTRY
With changing regulations and compliance issues, Pharmaceutical companies simultaneously face the challenges of reducing costs, raising the revenue and operational efficiencies, lower supply chain costs, whilst meeting regulatory and security requirements. Pharmaceutical companies can strategically make use of business intelligence software to make informed business decisions.
The business intelligence software can analyze, report and monitor vast amounts of data through business intelligence architecture and help companies reduce costs, increase revenue and maximize the value of information.
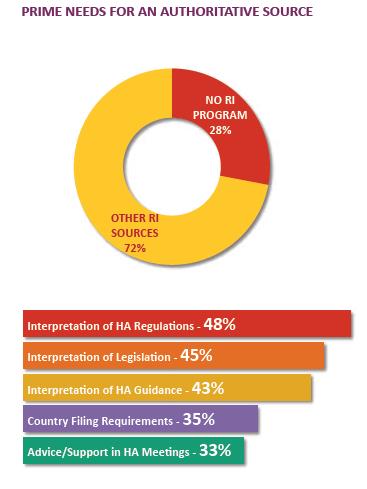
RI CHALLENGE FOR COMPANIES
Large and mid-tier companies with products in many markets face a definitive challenge in securing authoritative regulatory intelligence from regulatory intelligence groups. Companies presume the regulatory intelligence groups provide an authoritative interpretation of a wide range of national and regional regulations. In addition, the group must also provide an impact assessment of proposed regulatory changes in many more markets.
RI GROUPS: PAIN POINTS
In a 2014 survey about centralized RI programs, respondents placed high value but low satisfaction on the products of the RI group.
• The central RI program is usually located at the company headquarters and is managed
by a relatively small staff
• Work of the central RI group is often relatively broad coupled with the critical mission tasks
• Rising requirements for information and audits by Health Authorities
• 67% of the companies established a centralized regulatory intelligence office/program
• A quarter responded “yes” when asked if the centralized RI program is viewed as
the authoritative source for *hard and soft intelligence services
*Hard and soft intelligence services include core RI services like interpretation of laws, HA regulations and guidance.
REASONS FOR LESSER COMPANIES REPORTING RI GROUP AS THE AUTHORITATIVE SOURCE
• Use other internal experts to produce a complete analysis on regulations or guidance
• Some stakeholders rely on internal networks and external sources to develop an opinion and action plan to meet new regulatory requirements
COMPANIES ARE PLANNING A CHANGE IN
PROCESS: Improve internal communication among – central group, regulatory affiliates and functional areas
REGULATORY INFORMATION PROCESS MANAGEMENT TOOLS: Improve information access through portals, knowledge management systems and external tools
ROLES & RESPONSIBILITIES: Organizational changes –To identify and improve delivery to increase stakeholder satisfaction
REGULATORY INTELLIGENCE LONG TERM STRATEGIC BENEFITS
• Monitors regulatory environment and ensures compliance
• Saves time and money through real time intelligence gathering, analysis and dissemination
• Avoids risk of duplication and redundancies
• Addresses global language barrier with instant access to essential details in English
• Ensures accuracy of critical information obtained from distributors, manufacturers and other
industry contacts
• Maintains knowledge data bank by retaining and constantly adding to the regulatory knowledge
within the company
•Supports in creating a robust regulatory policy
• Reviews and helps update the old data with current regulatory trends
• Advises workforce of various newly evolving regulatory disciplines
• Provides detailed and customized insights for advance search
• Provides centralized and structured information management system
IN CONCLUSION
All companies perform regulatory intelligence to some extent and more companies are establishing dedicated intelligence groups. Regulatory intelligence scope does vary in form of active analysis and interpretation, however it does not equate to regulatory information.
Furthermore regulatory intelligence is imperative along with allocation of key tasks to ensure compliance, future awareness and adequate resourcing. Benefits of having the correct regulatory information will enable to design and implement a good regulatory strategy which can lead to reduced time-to market both via accelerated development and smoother registration assessment, reduced costs, increased compliance and ultimately optimization of return on investment.









