Colombia Medical Device Registration Overview
Colombia presents tremendous opportunities for medical device companies and its healthcare system is renowned for its commitment to patient safety and the quality of medical services and it governs medical devices through INVIMA (the National Institute for Food and Drug Surveillance). INVIMA is the operating authority for Colombia medical device registration.

Regulatory Authority: INVIMA (the National Institute for Food and Drug Surveillance)
Regulation: Decree 4725/2005
Regulatory Pathway: Device Registration
Authorized Representative: Colombia Legal Representative
QMS Requirement: ISO 13485:2016 / MDSAP
Assessment of Technical Data: INVIMA
Labeling Requirements: Labeling documents must be in Spanish language
Validity of License: 10 years
Submission Format: Electronic
Colombia Medical Device Classification
| Medical Device Classification | |
|---|---|
| Class I (Lowest risk) | |
| Class IIa | |
| Class IIb | |
| Class III (Highest risk) | |
Colombia Legal Representative
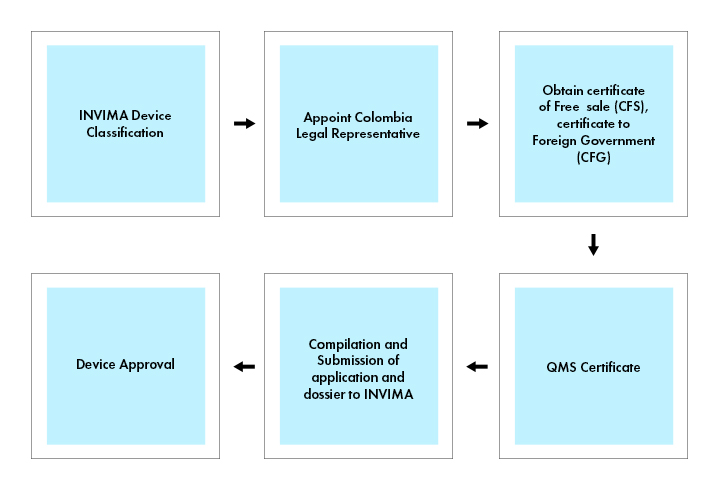
To be a Legal Representative in Colombia, one must either hold Colombian citizenship or possess the necessary legal authorization to reside and work in the country as a foreign national. A Legal Representative can assist and manage all registration procedures in Colombia, acting as a liaison between the company and INVIMA, the local regulatory authority.
Colombia Medical Device Registration
Medical Devices and IVDs are subject to registration procedure, so Medical Device must be registered with INVIMA to be marketed in Colombia.
To market a medical device in Colombia, it is required to obtain a Certificate of Free Sale (CFS), or a Certificate to Foreign Government (CFG) issued by the appropriate regulatory agency in the country of origin or a recognized reference country (for e.g., Canada, Japan, Australia, the European Union, and the United States). The Certificate of Free Sale or Certificate to Foreign Government, serves as evidence that the medical device meets the necessary safety and quality standards for sale in Colombia.
Process flow
Post Approval Device Life Cycle Management
Freyr supports the foreign manufacturers in end-to-end Medical Device lifecycle management, including post approval activities, such as:
- Post approval change management - modifications to existing medical device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the INVIMA and the manufacture
Summary
| Medical Device Classification | New licenses and Renewals | ||
|---|---|---|---|
| New registration | Amendment | Renewal | |
| Class I | 4-6 Months | 2-3 Months | 4-6 Months |
| Class IIa | 4-6 Months | 2-3 Months | 4-6 Months |
| Class IIb | 4-6 Months | 2-3 Months | 4-6 Months |
| Class III | 4-6 Months | 2-3 Months | 4-6 Months |
| Registration Fees | |||||
|---|---|---|---|---|---|
| New licenses and Renewals | Technical Modifications | Administrative Modifications | |||
| Class I & Class IIa | Class IIb & Class III | Class I & Class IIa | Class IIb & Class III | Class I & Class IIa | Class IIb & Class III |
| $ 690,00 | $ 776,00 | $ 196,00 | $ 196,00 | $ 153,00 | $ 153,01 |
Freyr Expertise
- Regulatory Due Diligence
- Official Classification
- Device Registration
- Colombia Registration Holder
- Labeling support
- Translation support
- Distributor identification and qualification
- Post Marketing Surveillance
- Post Approval Change Management
- License renewal and transfer
- Submission and liaison with the INVIMA