Medical Devices Regulatory Services
in Singapore Overview
Medical devices in Singapore are regulated under the Health Products Act (HPA) and its Health Product (Medical Devices) Regulations 2010.
Regulatory Authority: Health Science Authority (HSA)
Regulation: Health Products (Medical Devices) Regulations 2010
Regulatory Pathway: Medical Device Branch of the Health Sciences Authority (HSA)
Authorized Representative: Singapore Registrant
QMS Requirement: SS 620:2016, Singapore Standard for Good Distribution Practice for Medical Devices, and ISO 13485:2016
Assessment of Technical Data: Health Science Authority (HSA)
Validity of License: All dealer licenses are valid for 12 months from the date of approval
Labelling Requirements: Regulatory guidance, GN- 23 Revised (1 March 2020)
Submission Format: Online
Language: English
HSA Medical Device Classification
HSA applies 16 sets of rules to classify medical devices from lowest to highest risk into Class A, B, C, and D.
Risk classification depends on the factors like duration of contact, degree of invasiveness, intended use, and administration method.
Medical Device Classification, HSA
| Risk Class | Risk Level | Medical Device Examples |
|---|---|---|
| Class A | Low risk | Film viewer, surgical hand, sheath, oxygen mask |
| Class B | Low to moderate risk | Blood pressure cuff, stem sterilizer |
| Class C | Moderate to high risk | Patient monitor, mesin X-ray |
| Class D | High risk | Cardiac stents, pacemakers |
IVD Classification
In-Vitro Medical Devices are classified below from lowest to highest risk.
Risk class | Risk Level | In- Vitro Medical Device Examples |
|---|---|---|
| Class A | Low individual risk and low public health risk | Specimen receptacle |
| Class B | Moderate individual risk or low public health risk or both | Vitamin B12, pregnancy self-testing, anti-nuclear antibody, urine test strips |
| Class C | High individual risk or moderate public health risk or both | Blood glucose self-testing, HLA typing, PSA screening, Rubella IgM |
| Class D | High individual risk and high public health risk | HIV blood donor screening, HIV diagnostic kit |
Singapore Medical Device Authorized Representative / Registrant
A Registrant is the liaison between your company and HSA, who handles the device registration listing in Singapore. Singapore medical device authorized representative processes the registration application with the HSA and owns your HSA device registration. Only Singapore-based companies or entities can act as a Registrant; they must also be registered with the HSA.
HSA Medical Device Registration
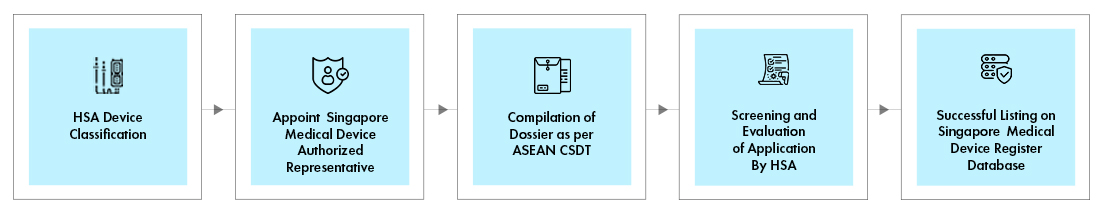
The HSA medical device registration process is conducted through the HSA online Medical Device Information and Communication system (MEDICS). Each HSA device registration is done via a specific evaluation route, depending on the following:
- Risk classification of the device.
- Number of prior approvals given by the overseas reference Regulatory agencies.
- Duration of safety marketing history of the device.
The evaluation route of the device will determine the Turn-Around-Time (TAT), fees, and documents required for registration.
Class A Registration - Class A medical devices are exempted from product registration. However, there is a need to complete the Class A exemption list in MEDICS during the dealer’s license application.
Process flow
Post Approval Device Life Cycle Management
Freyr supports foreign manufacturers in end-to-end medical device lifecycle management, including post-approval activities, such as:
- Post-approval change management - modifications to existing medical device approvals, such as the addition of new variants and accessories; the addition of new indications of use, among others.
- Maintenance of medical device approvals and registration through timely payment of administrative and registration fees.
- Renewal of license.
With a professional team to provide Regulatory support, Freyr supports manufacturers in maintaining the quality and safety needed for approval. Freyr’s intelligence experts keenly observe Regulatory updates and inform clients about steps to be taken for product compliance with prevalent standards.
Summary
The processing time for product registration is mentioned in the following table.
| Risk Class | Immediate | Expedited | Abridged | Full Evaluation | Full (Priority Review Scheme) |
|---|---|---|---|---|---|
| Class B | Immediate registration upon submission | 100 working days | 160 working days | 120 working days | |
| Class C | Immediate registration upon submission (for Class C standalone medical mobile application only) | 120 working days | 160 working days | 220 working days | 165 working days |
| Class D | 180 working days | 220 working days | 310 working days | 235 working days | |
| Class D (devices incorporating medicinal products) | 220 working days | 310 working days |
The turnaround time for a change of registrant is 40 working days.
NOTE-
- Class A medical devices are exempted from product registration.
- Turnaround time excludes the time taken to respond to any requests for clarification or additional information by HSA during the evaluation phase.
Freyr Expertise
- Regulatory Due Diligence for Device Registration with the HSA, Singapore
- HSA Medical Device Classification and Grouping
- Support for Conformity Assessment Body (CAB) Assessment
- ASEAN Common Submission Dossier Template (CSDT) Dossier Compilation
- HSA Device Registration;
- Legal Representation
- Labeling Support
- Distributor Identification and Qualification
- Post-marketing Surveillance
- Post-approval Change Management
- License Renewal and Transfer
- Submission and Liaising Services with the HSA