Argentina Medical Device Registration Overview
Argentina is one of the rapidly developing countries in Latin America that presents opportunities for medical device companies with a healthcare system known for its dedication to patient safety and high-quality medical services. Medical device registration in Argentina is overseen by the Regulatory authority – the National Administration of Drugs, Foods, and Medical Devices (ANMAT).

Regulatory Authority: National Administration of Drugs, Food and Medical Devices (ANMAT)
Regulations: Disposition 2318/2002, and Disposition 727/2013
Regulatory Pathway: Electronic Web Assisted Notification of Devices system (WAND)
Authorized Representative: Argentina Authorized Representative (AAR)
QMS Requirement: ISO 13485 (ANMAT MDS), and MDSAP (Affiliate Member)
Assessment of Technical Data: National Administration of Drugs, Food and Medical Devices (ANMAT)
Labeling Requirements: Annex III.B of Provision 2318/2002 and Annex V of Provision 727/2013.
Submission Format: Electronic (HELENA portal)
Language: Spanish
Medical Device Classification in Argentina
In the ANMAT Regulatory process, the first step in determining the registration path and compliance with Argentina regulations is to determine the classification of the medical device. In Argentina, devices are classified into four (04) classes based on the risk (Class I-IV).
| Medical Device Class | Criteria |
|---|---|
| Class I | Low Risk |
| Class II | Low - Moderate Risk |
| Class III | High - Moderate risk |
| Class IV | High Risk |
| IVD Class | Criteria |
|---|---|
| Class A | Diagnosis of non-infectious or non-communicable diseases. |
| Class B | Diagnosis of infectious diseases except those belonging to Class C. |
| Class C | Diagnosis of sexually transmitted infectious diseases, or transmitted by blood or its derivatives, as well as for identification of blood groups. |
| Class D | For self-assessment. |
Argentina Authorized Representative (AAR)
The registration holder of any medical device in Argentina is legally responsible for the registration and marketing of the device in the country. This can be the manufacturer of the device, or it can be a local authorized representative (AAR) in Argentina acting on behalf of the manufacturer.
All medical devices marketed in Argentina must be registered with the ANMAT. The registration process can be complex and time-consuming, so it is often recommended that foreign manufacturers appoint an AAR to assist them.
ANMAT Medical Device Registration
The ANMAT medical device registration process is similar to the Brazilian notification and registration processes, with the extent of scrutiny involved for approval increasing based on the device classification (Class I, II, III, and IV). All medical device manufacturers must also comply with the ANMAT-MDS, which is Argentina's equivalent of the BGMP and is also in line with ISO 13485:2016.
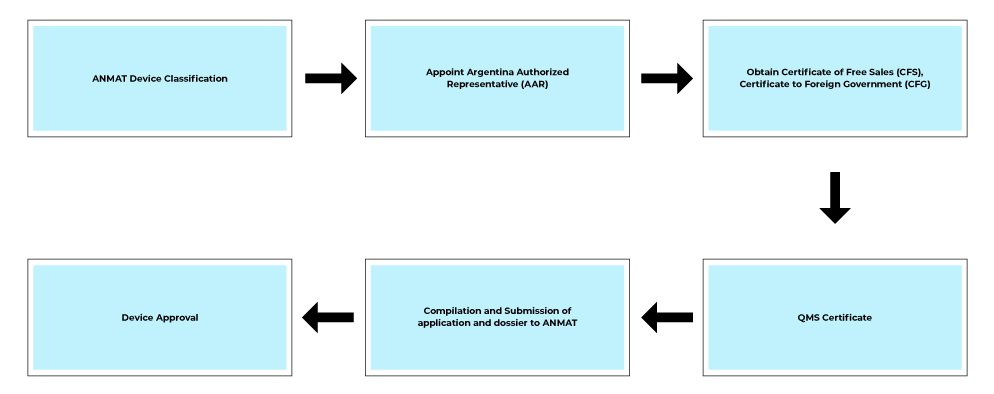
Process flow
Post-approval Services
Freyr supports foreign manufacturers with end-to-end medical device life cycle management, including post-approval activities, such as –
- Post-approval change management - modifications to existing medical device approvals, such as the addition of new variants, accessories, and addition of new indications of use, among others.
- Maintenance of approvals and registration through timely payment of administrative and registration fees.
- Renewal of licenses.
- Liaising between ANMATand the manufacturer.
- Adverse event reporting.
- Importation management.
Freyr assists manufacturers in identifying device classification as a first step. Freyr helps clients navigate the Regulatory pathway and procure certifications by helping rectify the Regulatory hassles involved. With proven expertise in launching numerous devices in the region, Freyr offers end-to-end Regulatory services for medical devices.
Summary
| Class of Devices | Registration Pathway (Notification Or Full Registration) | Health Agency Timelines | Validity of Registration (years) |
|---|---|---|---|
| Class I Devices | Full registration | 4- 6 months | 5 years |
| Class II Devices | Full registration | 4- 6 months | 5 years |
| Class III Devices | Full registration | 6-8 months | 5 years |
| Class IV Devices | Full registration | 6-8 months | 5 years |
*Please note that factors such as bandwidth limitations, regulatory updates, and other variables may lead to extensions in Health Authority timelines.
Freyr Expertise
- Regulatory Intelligence Report Services.
- Official Classification Services.
- Compilation of Technical Documents.
- Device Registration Services.
- Argentina Authorized Representative (AAR) Services.
- Importation Services.
- Operating Permits and Authorizations.
- Translation Services.
- Labeling & Artwork Services.
- Distributor Identification and Qualification Services.
- Post-market Surveillance.
- License Renewal and Transfer Services.
