Medical Device Registration in Pakistan Overview
To be sold in Pakistan, all medical devices must first be registered with the Drug Regulatory Authority of Pakistan (DRAP). DRAP protects patients by ensuring that devices fulfil quality standards, encourage public trust, and drive innovation with a clear path for safe and new technology.
Discover how Freyr's medical device Regulatory experts can streamline your Pakistan medical device consulting needs with comprehensive end-to-end services.
Pakistan Medical Device Classification:
| CLASS | LEVEL | DEVICE EXAMPLES |
| A | Low Hazard | Tongue depressors / disposable masks. |
| B | Low-moderate Hazard | Hypodermic Needles / suction equipment. |
| C | Moderate-high Hazard | Lung ventilator / bone fixation plate. |
| D | High Hazard | Heart valves / implantable defibrillator. |
Medical Device Registration in Pakistan:
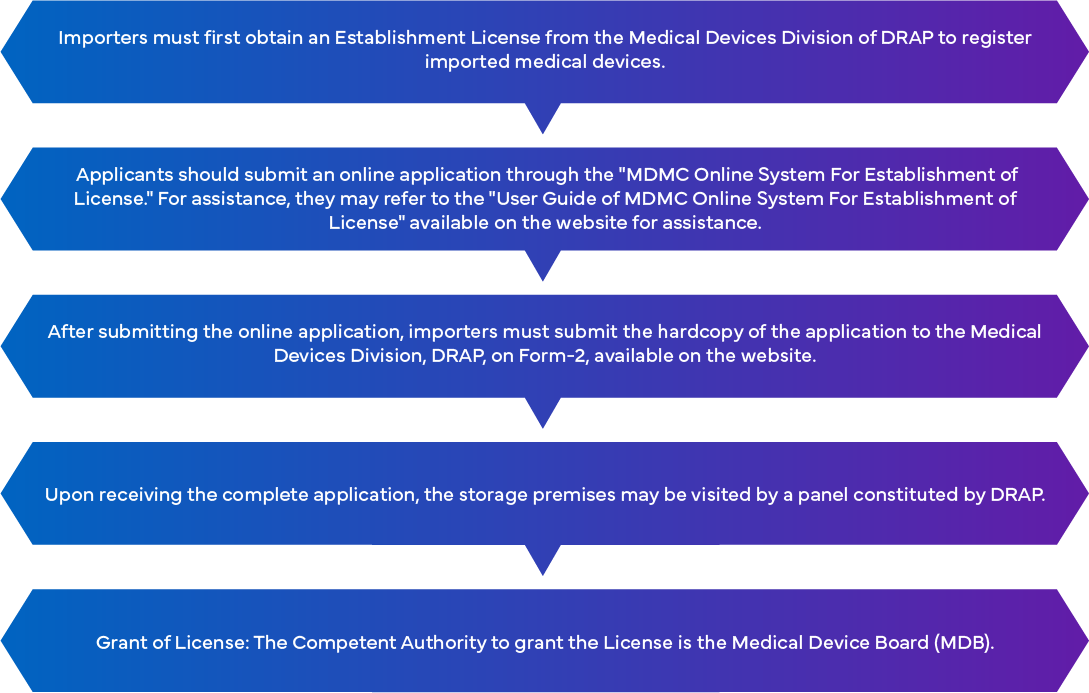
Pakistan Medical Device Registration Requirements for Market Entry
| Authority | Drug Regulatory Authority of Pakistan (DRAP) |
| Regulation | Drug Regulatory Authority of Pakistan (DRAP) vide S.R.O.32 (I)/2018, MDMC Online System for Establishment of License, |
| Classification | Class I (Lowest risk), Ia, IIb, and III (highest risk) |
| Pathway | Medical Device Board (MDB) of DRAP |
| Authorized Representative | Pakistan Market Authorization Holder |
| QMS Requirement | ISO 13485 Certification, Medical Device Rules, 2015 and DRAP Act, 2012 (GDPMD Certificate) |
| Validity of License | Five (05) Years |
| Labeling Requirements (Language) | English and Urdu |
Explore how Freyr can support your Pakistan medical device consulting needs with our expert services in medical device Regulatory affairs.
Freyr Competencies for Pakistan Market Entry
![]()
In-country Representation Foreign Manufacturer.![]()
Importation License Holding.![]()
Medical Devices / IVD Registration.![]()
Technical Documentations Evaluation and Registration.![]()
Regulatory Compliance Services.![]()
QMS/GDPMD Implementation & Compliance.![]()
Medical Devices Manufacturer License.![]()
Technical Advising.
Frequently Asked Questions (FAQs)
- Pakistan's Drug Regulatory Authority (DRAP) established a dedicated division, the Medical Device and Medicated Cosmetics Division.
- In 2015, this division introduced the country's first-ever Medical Devices Regulations.
- These regulations were later superseded by the Medical Device Rules 2017, which came into effect on January 16, 2018.
- The aim of these rules is to ensure public access to safe, effective, and high-quality medical devices.
- All Classes: ISO 13485, Letter of Authorization from Manufacturer.
- Class A (anyone): Free Sale Certificate, Declaration of Conformity, Production/Quality Assurance Certificate.
- Class B, C, or D (mandatory for Class D):
- Option A: Free Sale Certificate, Declaration of Conformity, CE Marking Certificate (Full Quality Assurance).
- Option B: Free Sale Certificate from Reference Countries (listed).
- Option C: Free Sale Certificate with WHO Prequalification.
The product registration dossier may be drafted in English.
Medical device labeling (labels and directions for usage) for professional use can be made available in English, however, labeling for household use must be in Urdu. In such situations, the identity data of the foreign manufacturer must be included alongside those of the Pakistan local authorized representative (Pakistan Market Authorization Holder) or importer.
Medical Device Regulatory Consulting – Proven Expertise
Why Freyr?
- Independent Representation and Regulatory Support for Medical Device Consulting in Pakistan.
- Continuous Liaison with the DRAP Pakistan for Submissions, Queries, and Feedback.
- Single Point of Contact (POC) in the Country for Liaison with the DRAP.
