
Vietnam Medical Device Registration Overview
Vietnam’s Medical Device market is picking up and is currently one of the booming sectors in the country. Medical Devices in the country are regulated by the Department of Medical Equipment and Health Works (DMEHW) under the Ministry of Health. Foreign manufacturers must appoint a Vietnam Local Authorized Representative to assist them in Vietnam medical device registration process.
Regulatory Authority: Department of Medical Equipment and Health Works (DMEHW)
Regulation: Decree no. 98/2021/ ND-CP
Authorized Representative: Vietnam Local Authorized Representative
QMS Requirement: ISO 13485:2016
Assessment of Technical Data: Department of Medical Equipment and Construction (DMEC) of MoH
Labelling Requirements: Decree no. 111/2021
Submission Format: Online- Timelines: 15 - 60 days
Language: English & Vietnamese
Vietnam Medical Device Classification
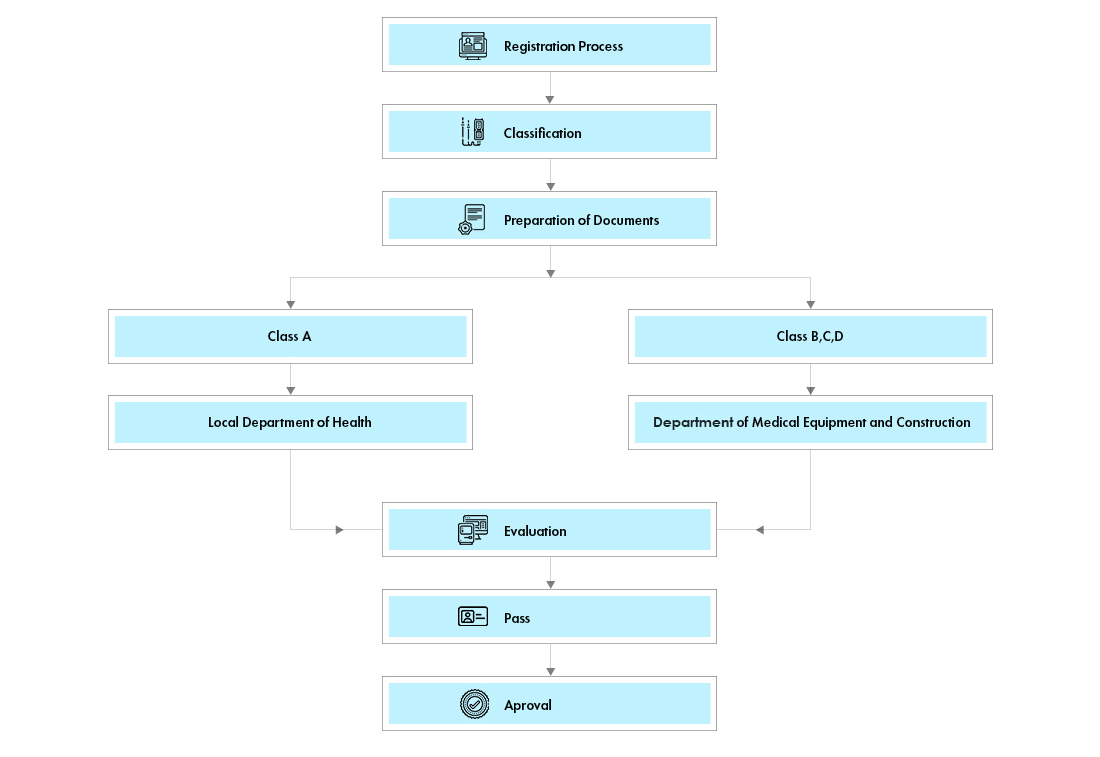
The devices are classified into 4 classes (A, B, C and D) which are also categorized into two groups, group 1 (Class A) and group 2 (Class B, C and D). Formal classification is available with the Vietnam Department of Medical Equipment and Health Works (DMEHW).
| Group | Class | Risk |
|---|---|---|
Group 1 | Class A | Low Level |
Group 2 | Class B | Lower average level of risk |
Class C | Upper average level of risks | |
Class D | High level of risks |
Vietnam Local Authorized Representative
Appointment of reliable and capable Vietnamese agent for foreign manufacturers is crucial as they must undertake the warranty services offered by manufacturer as part of the device sale. Translation to Vietnamese is a must to enter the region, which indeed can be challenging in actual practice.
Freyr offers Regulatory support extending the complete range of activities such as procuring Free-sale Recognition Number involved in Medical Device approval from authorities. We also provide linguistic expert support to maintain the Regulatory perspective of the translation intact. We cater to the post-approval needs of clients to maintain compliance throughout the product lifecycle in Vietnam.
Vietnam Medical Device Registration
All Class A and B products will require a Market Authorization (MA) License. Class A and B devices will undergo a quick administrative review by the Health Department of the province where the Registration Holder is located.
Class C and D devices will now need to apply for a Market Authorization (MA) license. MA licenses will remain valid indefinitely.
Process flow
Post Approval Device Life Cycle Management
Freyr supports the foreign manufacturers in end-to-end Medical Device lifecycle management, including post approval activities, such as:
- Post approval change management - modifications to existing Medical Device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the DMEHW and the manufacturer
- Importation management
Summary
Group | Class | Risk | Regulatory Pathway | Timelines | Validity |
|---|---|---|---|---|---|
Group 1 | Class A | Low Level | Declaration of applicable standard | 15 - 60 days | Unlimited |
Group 2 | Class B | Lower average level of risk | Certificate of free-sale registration | 15 - 60 days | 5 Years |
Class C | Upper average level of risks | Certificate of free-sale registration | 15 - 60 days | 5 Years | |
Class D | High level of risks | Certificate of free-sale registration | 15 - 60 days | 5 Years |
Freyr Expertise
- Regulatory Due-Diligence
- Official Classification
- Device Registration
- Import License
- Labeling support
- Translation support
- Distributor identification and qualification
- Post Marketing Surveillance
- Post Approval Change Management
- License renewal and transfer
- Submission and liaising
- Customs clearance


