About Us
Freyr is the largest global Regulatory solutions and services provider, offering end-to-end solutions to the Pharmaceutical, Medical Devices, and Consumer sectors. At Freyr, we specialize in Regulatory Affairs, Pharmacovigilance, Quality Management, and Technology Solutions for Submissions Management, Labeling Management, Regulatory Information Management, and Regulatory Intelligence.
With our deep industry expertise, global presence, and commitment to innovation, we empower clients to navigate complex Regulatory landscapes efficiently and effectively. From Regulatory strategy to submissions and compliance, we are your trusted partner in ensuring product success and patient safety.
+
+
+
+
Success Stories
Powering Progress: Real Regulatory Wins, Transforming Compliance into Success!

Freyr Facilitated Seamless Management of Over 70 ANDAs/NDAs for a US-based Pharmaceutical Company Through Comprehensive End-to-End Regulatory Support
A US-based pharmaceutical company had faced high attrition rates and limited in-house expertise, struggling with the Regulatory operations of 70+ ANDAs/NDAs. They had turned to Freyr for comprehensive Regulatory services. Freyr had provided strategic guidance and submission management, transforming the company's Regulatory operations. This support ensured seamless operations, timely approvals, and enhanced compliance.
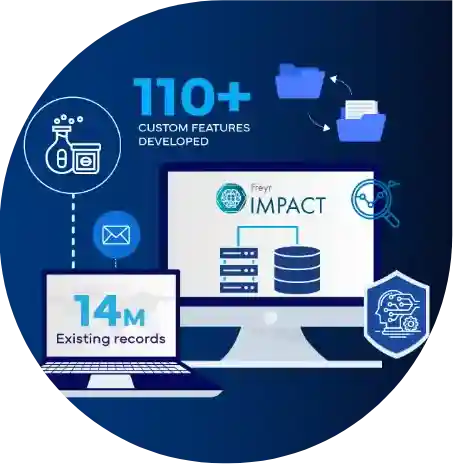
Freyr transformed the Regulatory Intelligence Ecosystem in a Global Bio-Pharmaceutical Company by building a World-class, Custom Regulatory Intelligence product
Join us on a remarkable journey as Freyr transforms the Regulatory Intelligence ecosystem for a top 5 global bio-pharmaceutical giant. Faced with fragmented Regulatory and decision-making processes, our custom solution integrated advanced technologies to centralize their records and streamline global compliance tracking, truly transforming their Regulatory intelligence processes.
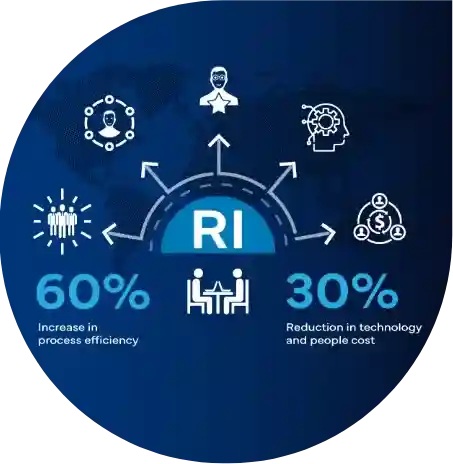
Freyr leads the establishment of a new Regulatory Intelligence function within a Large Size Innovator Pharmaceutical Entity through Bespoke Staffing solution and Consultancy services
Uncover the story of a prominent EU pharmaceutical innovator grappling with Regulatory intelligence hurdles. Drowning in data from multiple tools, they struggled with inefficient processes and reactive decision-making. Freyr's tailored 360-degree consulting approach came in as a complete game-changer, significantly boosting efficiency and reducing operational costs.

Freyr Managed 15+ New and Approved Products (ANDAs/NDAs) for a Leading US Pharmaceutical Company with Comprehensive Regulatory Operations Support
A leading US pharmaceutical company had faced significant challenges in managing over 15 new and approved products (ANDAs/NDAs) due to a lack of in-house Regulatory expertise and limited FDA knowledge. Seeking comprehensive Regulatory support, they had turned to Freyr. Freyr's expert team provided strategic guidance, submission support, and ongoing Regulatory operations management, offering end-to-end Regulatory solutions that ensured seamless submission processes and continuous Regulatory compliance. As a result of Freyr's support, the pharmaceutical company achieved zero rejections and maintained Regulatory excellence.

Freyr supported a German-based Pharma and Medical Device leader with technical writing services throughout the Device Lifecycle of their SaMD radiology solution
The customer required Freyr to migrate content from the legacy application (FrameMaker) to Vasont Inspire Component Content Management System (CCMS). Freyr tailored Technical Writing solutions enabled the customer to achieve their goal with a 40% quicker turn-around-time.

Freyr Facilitated Seamless Management of Over 70 ANDAs/NDAs for a US-based Pharmaceutical Company Through Comprehensive End-to-End Regulatory Support
A US-based pharmaceutical company had faced high attrition rates and limited in-house expertise, struggling with the Regulatory operations of 70+ ANDAs/NDAs. They had turned to Freyr for comprehensive Regulatory services. Freyr had provided strategic guidance and submission management, transforming the company's Regulatory operations. This support ensured seamless operations, timely approvals, and enhanced compliance.

Freyr transformed the Regulatory Intelligence Ecosystem in a Global Bio-Pharmaceutical Company by building a World-class, Custom Regulatory Intelligence product
Join us on a remarkable journey as Freyr transforms the Regulatory Intelligence ecosystem for a top 5 global bio-pharmaceutical giant. Faced with fragmented Regulatory and decision-making processes, our custom solution integrated advanced technologies to centralize their records and streamline global compliance tracking, truly transforming their Regulatory intelligence processes.

Freyr leads the establishment of a new Regulatory Intelligence function within a Large Size Innovator Pharmaceutical Entity through Bespoke Staffing solution and Consultancy services
Uncover the story of a prominent EU pharmaceutical innovator grappling with Regulatory intelligence hurdles. Drowning in data from multiple tools, they struggled with inefficient processes and reactive decision-making. Freyr's tailored 360-degree consulting approach came in as a complete game-changer, significantly boosting efficiency and reducing operational costs.

Freyr Managed 15+ New and Approved Products (ANDAs/NDAs) for a Leading US Pharmaceutical Company with Comprehensive Regulatory Operations Support
A leading US pharmaceutical company had faced significant challenges in managing over 15 new and approved products (ANDAs/NDAs) due to a lack of in-house Regulatory expertise and limited FDA knowledge. Seeking comprehensive Regulatory support, they had turned to Freyr. Freyr's expert team provided strategic guidance, submission support, and ongoing Regulatory operations management, offering end-to-end Regulatory solutions that ensured seamless submission processes and continuous Regulatory compliance. As a result of Freyr's support, the pharmaceutical company achieved zero rejections and maintained Regulatory excellence.

Freyr supported a German-based Pharma and Medical Device leader with technical writing services throughout the Device Lifecycle of their SaMD radiology solution
The customer required Freyr to migrate content from the legacy application (FrameMaker) to Vasont Inspire Component Content Management System (CCMS). Freyr tailored Technical Writing solutions enabled the customer to achieve their goal with a 40% quicker turn-around-time.

Freyr Facilitated Seamless Management of Over 70 ANDAs/NDAs for a US-based Pharmaceutical Company Through Comprehensive End-to-End Regulatory Support
A US-based pharmaceutical company had faced high attrition rates and limited in-house expertise, struggling with the Regulatory operations of 70+ ANDAs/NDAs. They had turned to Freyr for comprehensive Regulatory services. Freyr had provided strategic guidance and submission management, transforming the company's Regulatory operations. This support ensured seamless operations, timely approvals, and enhanced compliance.
Redefining Regulatory Excellence
Take advantage of our integrated suite of services, which range from end-to-end Regulatory consultation, process automation with robust Regulatory software, and harnessing the power of Artificial Intelligence (AI) in Regulatory intelligence, to transforming market entry challenges into growth opportunities.
Global Regulatory Services
Navigate Regulatory complexities effortlessly with our end-to-end global services. From strategy to compliance, we've got you covered globally. Ensure success in every market with Freyr Solutions.
Regulatory Software
Elevate your Regulatory efficiency with Freyr Regulatory Software. Streamline processes, ensure compliance, and conquer global markets seamlessly with our advanced Regulatory solutions.
Global Regulatory Intelligence
Stay ahead of the ever-evolving regulatory landscape with Freyr GRI. Powered by AI, NLP and ML technologies, our tailored and data-driven insights empower you to make informed strategic decisions with confidence and ease.
Your AI-Enabled Regulatory Wiz by Freyr
Celebrating Customers Success
We are proud partners to a diverse range of industry leaders. Our commitment to excellence in Regulatory services has earned us
the trust of esteemed organizations.
Cosmetics
Toxicological Safety Assessment Reports
Vietnam
Thank you so much for being a great partner in our regulatory compliance journey. As ASEAN countries move towards having safety assessments as a key requirement, your support has helped us significantly in fulfilling those requirements well ahead of our competitors in Vietnam.
In fact, I shared your contact to our regulatory officers so that they can share it across the industry if the safety assessments become a must.”
RA Assistant Manager
India-based, Multinational Consumer Goods Company
Food Supplements
Freyr exceeded our expectations by delivering a hassle-free and smooth product registration experience in the EU. Their team was professional, responsive, and always ready to provide clarity when needed. As a result, we are now confidently operating in five EU countries with our dietary supplements, thanks to their expert guidance and flawless execution. We highly recommend Freyr for regulatory support.

Bien Almonte
QC & Regulatory Manager
Cosmetics
End-to-end regulatory services
Global
It has been a pleasure working with Freyr, thanks to the seamless project delivery and the value your team consistently brought to the table. The expertise and professionalism demonstrated have exceeded expectations. I look forward to future opportunities to collaborate.

KASIA FRANKOWSKA
Head of Regulatory Affairs
Medical Devices
Registration and LR Support
Global
Freyr has been an indispensable partner in achieving rapid global scalability for our Software as a Medical Device (SaMD) business. As a startup, acquiring expertise in worldwide regulations is cost-prohibitive. Freyr's competitive pricing and tailored services allowed us to get that expertise at a fraction of the cost of full-time resources. Their team's responsiveness and adaptability to project priorities have greatly facilitated our progress. We recommend Freyr to any company seeking expert guidance and support in the medical device Regulatory domain.

Arie Henkin
VP - Quality and Regulatory, Australia -based, Leading SaMD Company
Medicinal Products
Publishing and Submission
UK
We would like to appreciate Freyr’s quick TAT to push through an urgent submission required by the FDA. Their efficiency, diligence, excellence, sense of urgency, and priority are deeply flexibility.
Please keep up the great work as we have many milestones to achieve over the next year.

Ed Venkat
Global CMC Technical Lead
Medical Devices
Swiss Rep Services
Japan and Switzerland
I genuinely enjoy my time working with Freyr, and I view them as a truly valuable asset and extension to my own team. They are dependable and accurate, and their pricing is competitive. Beyond that, I won’t hesitate to collaborate with Freyr again.

Darren Mansell
Regulatory Affairs Manager, UK-based, Global Medical Device Design and Manufacturing Company
Medicinal Products
Regulatory Affairs
USA
Please relate to the team the excellent job they have done on the IND reactivation. Specifically, the high-quality module 3, written by Freyr. It was very comprehensive, very few revisions were required, and we had no questions from Health Canada or FDA. No CMC questions: that was a first for me. So very responsive to my many requests, which I am sure can be frustrating but always helpful while producing excellent work.
Thank you for always being available and responding quickly and comprehensively to all my requests.
What a great team you have, Freyr.

Lynne McGrath
Regulatory Consultant
Medical Devices
Registration and AR Services
Malaysia and Indonesia
Freyr provides a reliable service with expertise across many countries. I can rely upon Freyr to provide the information necessary to make an informed decision before entering a formal scope of work agreement. Once a project is underway, the Freyr team acts professionally to execute the work with excellent communication of progress.

Robert Menadue
Regulatory and Quality Assurance Manager, Australia-based, Medical Device Manufacturing and Distribution Company
Medicinal Products
Publishing and Submission
UK
I am sure you have heard by now that we have received our first-ever approval from the FDA for our Brands division. This is a major milestone for us as well as for my team here in Reg Ops. I would be remiss if I didn’t point out that we would not have been able to do this without the help of your dedicated team. From the original filing, then the following year of responses, all helped get us to final approval.
Thank you Freyr team for a job well done!

Michael Bellero
Sr. Director, Head of Regulatory Operations
Medical Devices
Registration and LR Services
Brazil
We are impressed with Freyr’s support in providing us with quick and well-detailed solutions to our queries. Freyr’s constant support to adapt to ever-changing Regulatory conditions while providing support with any additional queries we had in a timely manner has truly impressed us.

Sergey Burlov
Quality Manager, Russia-based, Innovative SaMD Company
Cosmetics
End-to-End Notification Services
Indonesia, Philippines, Thailand
We love working with you and have been very impressed with your team, so we plan on introducing our own branded products (or other white-label products) in Indonesia with you in the future.
Partner
US-based, Leading Cosmetics Manufacturing Company
Medical Devices
UKRP Support
UK
FREYR has accompanied us with the registration of several products on the UK market. They have always been quick to reply, attentive to our needs, a great source of Regulatory information and support. The price is reasonable compared with other similar service providers. We particularly appreciate the personalized quarterly and annual status reports that Freyr provides. When we call on FREYR, we know they will do their best to satisfy our needs, and that customer satisfaction is a priority.

Pascale LE BAUD
Regulatory Affairs Associate - RA Department, France-based, Leading Synthetic Implants Manufacturing Company
Food Supplements
Product Compliance
India
It has been a pleasure to work with Freyr. The team played a big part in launching out product in India. I thank everyone on the team.
Director
US-based, Leading Nutritional and Herbal Supplements Manufacturing Company
Food Supplements
FSSAI Registration
India
Thank you Freyr! Your SME was a star during this whole process. What you did for us is hugely appreciated. I am looking forward to continuing to work with Freyr. So, thanks you everyone on the team at Freyr.
Director
US-based, Leading Nutritional and Herbal Supplements Manufacturing Company
Cosmetics
Regulatory Competitive Intelligence
UK
Thank you for reaching out and letting me express the awesome work that Freyr provided for both projects that met the cost and project deliverables. Outstanding Freyr Team! You guys rocked! We are delighted with the outcome of these, and we are in the process of sharing them with our management and our department. I want to especially give a shout-out to Freyr's resource, as she provided outstanding technical leadership and output that met our expectations. With that said, we are looking forward to partnering with you for future projects.
Global Regulatory Affairs, Discovery
US-based, Leading Consumer Health Care MLM Company
Chemicals
Training Manuals Project Update
Global
Congratulation to the entire Freyr Solution team!!!!
It has been a complex exercise for both parties, and I am so glad we got to a successful end.
As you can imagine, we are facing a new situation due to COVID-19. We are focused on delivering business targets and to establish new necessary ways of working.
Group Leader
US-based, Multinational Food and Beverage Company
Food Supplements
Immunity Boosting Supplement
USA
It was indeed a pleasure having availed your service and we are happy that you could guide us through and complete the label design of our product.
We are thankful to you!
General Manager
India-based, Global Dietary Supplements Company
Cosmetics
End-to-End Product Registration
India
Thank you so much for sharing good news and registration certificates. With your prompt follow-up and support, we received registration certificates so much earlier than we initially expected!
We appreciate your services and professionalism. And I hope we will work on new projects with you very soon.
Manager International Business
South Korea-based, Leading Export & Import Services Company
Chemicals
Product Compliance
Global
I wanted to wish you all well and reiterate my and the team's thanks for all your hard work and efforts. I think that we are really moving into a position now where we have clear ways of working and a more defined plan. All the best, and I hope to work with you again in the future.
Regulatory Compliance Scholl Associate- FLP, Research and Development
UK-based, Multinational Consumer Goods Company
Food Supplements
Strategic Consulting
Japan, Thailand
I think these reports are very detailed and generally either confirmed some of our assumptions or provided some new context around claims or administrative practices that helped complement our understanding. Thank you so much for your diligence towards these.
Global Regulatory Director, VMS
UK-based, Multinational Consumer Goods Company
Cosmetics
Toxicological Safety Assessment Reports
Vietnam
Thank you so much for being a great partner in our regulatory compliance journey. As ASEAN countries move towards having safety assessments as a key requirement, your support has helped us significantly in fulfilling those requirements well ahead of our competitors in Vietnam.
In fact, I shared your contact to our regulatory officers so that they can share it across the industry if the safety assessments become a must.”
RA Assistant Manager
India-based, Multinational Consumer Goods Company
Food Supplements
Freyr exceeded our expectations by delivering a hassle-free and smooth product registration experience in the EU. Their team was professional, responsive, and always ready to provide clarity when needed. As a result, we are now confidently operating in five EU countries with our dietary supplements, thanks to their expert guidance and flawless execution. We highly recommend Freyr for regulatory support.

Bien Almonte
QC & Regulatory Manager
Cosmetics
End-to-end regulatory services
Global
It has been a pleasure working with Freyr, thanks to the seamless project delivery and the value your team consistently brought to the table. The expertise and professionalism demonstrated have exceeded expectations. I look forward to future opportunities to collaborate.

KASIA FRANKOWSKA
Head of Regulatory Affairs
Medical Devices
Registration and LR Support
Global
Freyr has been an indispensable partner in achieving rapid global scalability for our Software as a Medical Device (SaMD) business. As a startup, acquiring expertise in worldwide regulations is cost-prohibitive. Freyr's competitive pricing and tailored services allowed us to get that expertise at a fraction of the cost of full-time resources. Their team's responsiveness and adaptability to project priorities have greatly facilitated our progress. We recommend Freyr to any company seeking expert guidance and support in the medical device Regulatory domain.

Arie Henkin
VP - Quality and Regulatory, Australia -based, Leading SaMD Company
Medicinal Products
Publishing and Submission
UK
We would like to appreciate Freyr’s quick TAT to push through an urgent submission required by the FDA. Their efficiency, diligence, excellence, sense of urgency, and priority are deeply flexibility.
Please keep up the great work as we have many milestones to achieve over the next year.

Ed Venkat
Global CMC Technical Lead
Medical Devices
Swiss Rep Services
Japan and Switzerland
I genuinely enjoy my time working with Freyr, and I view them as a truly valuable asset and extension to my own team. They are dependable and accurate, and their pricing is competitive. Beyond that, I won’t hesitate to collaborate with Freyr again.

Darren Mansell
Regulatory Affairs Manager, UK-based, Global Medical Device Design and Manufacturing Company
Medicinal Products
Regulatory Affairs
USA
Please relate to the team the excellent job they have done on the IND reactivation. Specifically, the high-quality module 3, written by Freyr. It was very comprehensive, very few revisions were required, and we had no questions from Health Canada or FDA. No CMC questions: that was a first for me. So very responsive to my many requests, which I am sure can be frustrating but always helpful while producing excellent work.
Thank you for always being available and responding quickly and comprehensively to all my requests.
What a great team you have, Freyr.

Lynne McGrath
Regulatory Consultant
Medical Devices
Registration and AR Services
Malaysia and Indonesia
Freyr provides a reliable service with expertise across many countries. I can rely upon Freyr to provide the information necessary to make an informed decision before entering a formal scope of work agreement. Once a project is underway, the Freyr team acts professionally to execute the work with excellent communication of progress.

Robert Menadue
Regulatory and Quality Assurance Manager, Australia-based, Medical Device Manufacturing and Distribution Company
Medicinal Products
Publishing and Submission
UK
I am sure you have heard by now that we have received our first-ever approval from the FDA for our Brands division. This is a major milestone for us as well as for my team here in Reg Ops. I would be remiss if I didn’t point out that we would not have been able to do this without the help of your dedicated team. From the original filing, then the following year of responses, all helped get us to final approval.
Thank you Freyr team for a job well done!

Michael Bellero
Sr. Director, Head of Regulatory Operations
Medical Devices
Registration and LR Services
Brazil
We are impressed with Freyr’s support in providing us with quick and well-detailed solutions to our queries. Freyr’s constant support to adapt to ever-changing Regulatory conditions while providing support with any additional queries we had in a timely manner has truly impressed us.

Sergey Burlov
Quality Manager, Russia-based, Innovative SaMD Company
Cosmetics
End-to-End Notification Services
Indonesia, Philippines, Thailand
We love working with you and have been very impressed with your team, so we plan on introducing our own branded products (or other white-label products) in Indonesia with you in the future.
Partner
US-based, Leading Cosmetics Manufacturing Company
Medical Devices
UKRP Support
UK
FREYR has accompanied us with the registration of several products on the UK market. They have always been quick to reply, attentive to our needs, a great source of Regulatory information and support. The price is reasonable compared with other similar service providers. We particularly appreciate the personalized quarterly and annual status reports that Freyr provides. When we call on FREYR, we know they will do their best to satisfy our needs, and that customer satisfaction is a priority.

Pascale LE BAUD
Regulatory Affairs Associate - RA Department, France-based, Leading Synthetic Implants Manufacturing Company
Food Supplements
Product Compliance
India
It has been a pleasure to work with Freyr. The team played a big part in launching out product in India. I thank everyone on the team.
Director
US-based, Leading Nutritional and Herbal Supplements Manufacturing Company
Food Supplements
FSSAI Registration
India
Thank you Freyr! Your SME was a star during this whole process. What you did for us is hugely appreciated. I am looking forward to continuing to work with Freyr. So, thanks you everyone on the team at Freyr.
Director
US-based, Leading Nutritional and Herbal Supplements Manufacturing Company
Cosmetics
Regulatory Competitive Intelligence
UK
Thank you for reaching out and letting me express the awesome work that Freyr provided for both projects that met the cost and project deliverables. Outstanding Freyr Team! You guys rocked! We are delighted with the outcome of these, and we are in the process of sharing them with our management and our department. I want to especially give a shout-out to Freyr's resource, as she provided outstanding technical leadership and output that met our expectations. With that said, we are looking forward to partnering with you for future projects.
Global Regulatory Affairs, Discovery
US-based, Leading Consumer Health Care MLM Company
Chemicals
Training Manuals Project Update
Global
Congratulation to the entire Freyr Solution team!!!!
It has been a complex exercise for both parties, and I am so glad we got to a successful end.
As you can imagine, we are facing a new situation due to COVID-19. We are focused on delivering business targets and to establish new necessary ways of working.
Group Leader
US-based, Multinational Food and Beverage Company
Food Supplements
Immunity Boosting Supplement
USA
It was indeed a pleasure having availed your service and we are happy that you could guide us through and complete the label design of our product.
We are thankful to you!
General Manager
India-based, Global Dietary Supplements Company
Cosmetics
End-to-End Product Registration
India
Thank you so much for sharing good news and registration certificates. With your prompt follow-up and support, we received registration certificates so much earlier than we initially expected!
We appreciate your services and professionalism. And I hope we will work on new projects with you very soon.
Manager International Business
South Korea-based, Leading Export & Import Services Company
Chemicals
Product Compliance
Global
I wanted to wish you all well and reiterate my and the team's thanks for all your hard work and efforts. I think that we are really moving into a position now where we have clear ways of working and a more defined plan. All the best, and I hope to work with you again in the future.
Regulatory Compliance Scholl Associate- FLP, Research and Development
UK-based, Multinational Consumer Goods Company
Food Supplements
Strategic Consulting
Japan, Thailand
I think these reports are very detailed and generally either confirmed some of our assumptions or provided some new context around claims or administrative practices that helped complement our understanding. Thank you so much for your diligence towards these.
Global Regulatory Director, VMS
UK-based, Multinational Consumer Goods Company
Cosmetics
Toxicological Safety Assessment Reports
Vietnam
Thank you so much for being a great partner in our regulatory compliance journey. As ASEAN countries move towards having safety assessments as a key requirement, your support has helped us significantly in fulfilling those requirements well ahead of our competitors in Vietnam.
In fact, I shared your contact to our regulatory officers so that they can share it across the industry if the safety assessments become a must.”
RA Assistant Manager
India-based, Multinational Consumer Goods Company
Functional Delivery Models
Shaping the future of Regulatory excellence!
Let Us Innovate and Succeed Together
Connect with Freyr, your Gateway to Regulatory Excellence!
Time is of the essence as we navigate the intricate world of regulations. Take control of your compliance journey today and allow our team of experts to guide you toward success. With our customized solutions, you would always be ahead in the constantly evolving Regulatory landscape, ensuring the growth and sustainability of your business. Let's collaborate and succeed together.