The National Institute of Food and Drug Safety Evaluation (NIFDS) is the leading South Korean agency responsible for the scientific evaluation of food and drug safety. Its primary objective is to ensure food and drug safety by conducting scientific and expert reviews, approvals, risk assessments, investigations, and research. Its mission is to enhance public health and well-being through the scientific safety evaluation of food and pharmaceuticals.
A Comprehensive Overview of the NIFDS
The NIFDS is divided into six (06) departments, each with its own set of duties and responsibilities. The departments collaborate on research and development activities to enhance the institute’s capabilities for promoting the safety of food and medicines and safeguarding public health. The NIFDS’s six (06) departments, along with their hierarchy, are illustrated in Figure 1 below:
Figure 1: The Six (06) Departments of the NIFDS
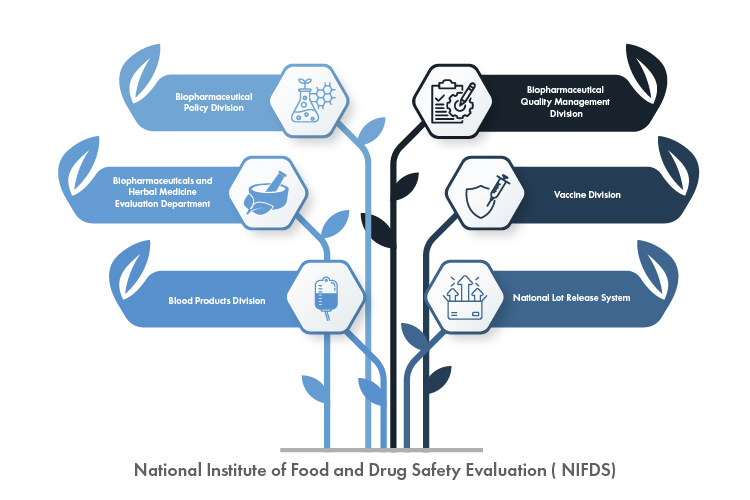
Here is a closer look at the functions of each division of NIFDS:
- Biopharmaceutical Policy Division: This division is in charge of developing and executing biopharmaceutical policies, thereby ensuring that the Regulatory framework is up to date with the newest advancements in the field.
- Biopharmaceutical Quality Management Division: This division is responsible for maintaining and improving the quality standards of biopharmaceutical products, thereby contributing to their safety and efficacy.
- Biopharmaceutical and Herbal Medicine Evaluation Department: This department is responsible for evaluating biopharmaceuticals and herbal medicines to ensure that they meet the essential safety and efficacy requirements before they are licensed for usage.
- Vaccine Division: This division is in charge of vaccine evaluation and regulation, thus playing a significant role in guaranteeing the safety and efficacy of these vital medical products.
- Blood Product Division: This division is in charge of evaluating and regulating blood products, thus ensuring the safety and quality of blood-based medical interventions.
- National Lot Release System: This system is in charge of releasing products that are either domestically manufactured or imported, thus verifying that they ulfil all the requirements before being sold in the market.
The key roles and responsibilities of the NIFDS are as follows:
- Research and Development: The NIFDS partners with ministries and institutes to increase its research capacity and strengthen the Ministry of Food and Drug Safety’s (MFDS) ability to promote health and well-being.
- Policy Research Study and Technical Evaluation: The NIFDS is in charge of conducting policy research studies and technical evaluations of pre-market approval applications.
- Review, Testing, and Analyses: The NIFDS is responsible for conducting reviews, tests, and analyses related to food and drug safety.
- Advanced Regulatory Science Research on MFDS-regulated Products: The NIFDS participates in advanced Regulatory science research on MFDS-regulated products, with an emphasis on public health and patient relief.
- International Collaboration: The NIFDS collaborates with both domestic and foreign research institutes to enhance the MFDS’s capacity to promote health and well-being.
Thus, the NIFDS stands as a cornerstone in South Korean Regulatory science, offering vital scientific evidence and expertise in food and drug safety management. Pharmaceutical businesses navigating South Korea’s intricate Regulatory landscape must partner with Regulatory service providers. Elevate your compliance journey with Freyr – your trusted ally in Regulatory excellence. Contact us today to ensure seamless adherence to NIFDS’s laws and regulations!