Non-clinical research is the foundation of drug product development, providing critical data on the safety and efficacy of new compounds. This stage is crucial as it sets the groundwork for clinical trials and eventual drug approval. However, non-clinical research faces numerous challenges that can impact the overall development timeline and success. This blog explores these challenges and offers solutions, highlighting the role of Regulatory partners in navigating these complexities.
Key Challenges and Solutions
Animal Model Variability
Challenge: Ensuring consistency and relevance of animal models to human biology is one of the primary challenges in non-clinical research. Variability in animal models can lead to inconsistent results, making it difficult to predict human responses accurately.
Solution: Using genetically consistent strains and appropriate models for the study can mitigate this issue. Selecting animal models that closely mimic human disease conditions and physiological responses ensures more reliable and translatable data.
Data Reproducibility
Challenge: Data reproducibility is critical for validating findings and ensuring that results can be replicated in different settings. Inconsistent protocols and a lack of standardized procedures can lead to variability in results.
Solution: Implementing robust protocols and validation techniques is essential. Standardized procedures, rigorous quality control measures, and thorough documentation can enhance the reproducibility and reliability of the data.
Regulatory Compliance
Challenge: Staying updated with evolving Regulatory guidelines and ensuring all studies meet required standards is a continuous challenge. Noncompliance can result in delays, additional testing, and even rejection of drug candidates.
Solution: Regular training and updates on Regulatory requirements are crucial. Engaging with Regulatory experts who have a deep understanding of global Regulatory landscapes can help ensure compliance and streamline the approval process.
Ethical Considerations
Challenge: Adhering to ethical standards and guidelines for animal research is paramount to ensure humane treatment. Ethical lapses can lead to public backlash, Regulatory penalties, and compromised study integrity.
Solution: Strict adherence to ethical guidelines and humane treatment of animals is non-negotiable. Establishing Institutional Animal Care and Use Committees (IACUCs) and following the 3Rs principle (Replacement, Reduction, Refinement) can ensure ethical compliance.
Table 1: Key Challenges and Solutions in Non-clinical Research
| Challenges | Solutions |
|---|---|
| Animal Model Variability | Use of genetically consistent strains and appropriate models |
| Data Reproducibility | Standardized protocols and validation techniques |
| Regulatory Compliance | Regular training and updates on Regulatory requirements |
| Ethical Considerations | Adherence to ethical guidelines and humane treatment of animals |
The Role of Regulatory Partners
Regulatory partners play a pivotal role in navigating the challenges of non-clinical research. They offer expertise in:
- Strategic Advice: Guiding study design, protocol development, and Regulatory submissions.
- Compliance Assurance: Ensuring studies meet global Regulatory standards and guidelines.
- Data Interpretation: Assisting in the analysis and presentation of non-clinical data to support Regulatory submissions.
- Ethical Oversight: Advising on ethical considerations and ensuring humane treatment of animal models.
Regulatory partners help in preparing comprehensive reports for submission and addressing Regulatory queries effectively. Their involvement can streamline the research process, reduce risks, and enhance the likelihood of successful drug development.
Benefits of Partnering with Regulatory Experts
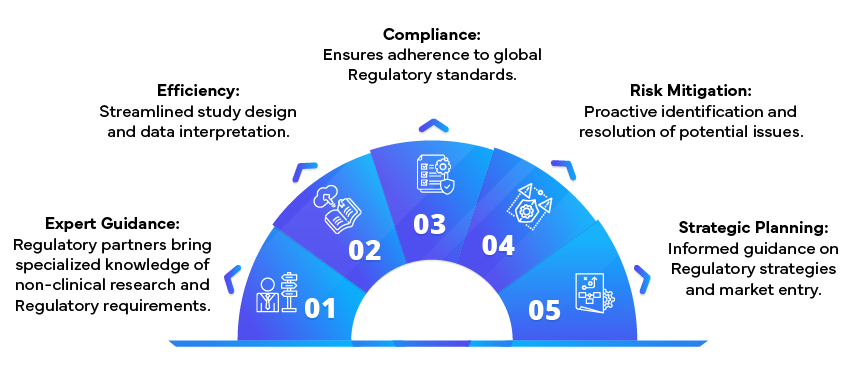
Addressing challenges in non-clinical research is crucial for successful drug product development. By implementing robust protocols, adhering to ethical standards, and staying updated with Regulatory requirements, pharmaceutical companies can overcome these hurdles. Partnering with Regulatory experts further enhances the quality and compliance of non-clinical research, ensuring that drug candidates progress smoothly through the development pipeline.