
September 24th 2016; with the deadline, just a month away for Class II device Unique Device Identifier (UDI) compliance, it is our assumption, if not for sure, that all medical device manufacturers are well equipped with a comprehensive Regulatory roadmap. Besides having a firm grip over governance pre-requisites, manufacturers are expected to be audit ready for the compliance. Right from validating Device Identifier (DI) and Product Identifier (PI) records to successful GUDID submissions, they are ought to be knowledgeable on the data to be submitted and procedures to be followed. Either pre-submission or post-submission, equipped with better knowledge on procedural know-how might protect manufacturers from corresponding challenges pertaining to data aggregation, submitting DI records and managing device reports, tracking HA acknowledgements etc.
To equip manufacturers with a better stand in such scenarios, here we provide a quick-to understand UDI Compliance challenges vs solutions infographic, which might save your time to decode compliance complexities and better plan ahead and structure the roadmap
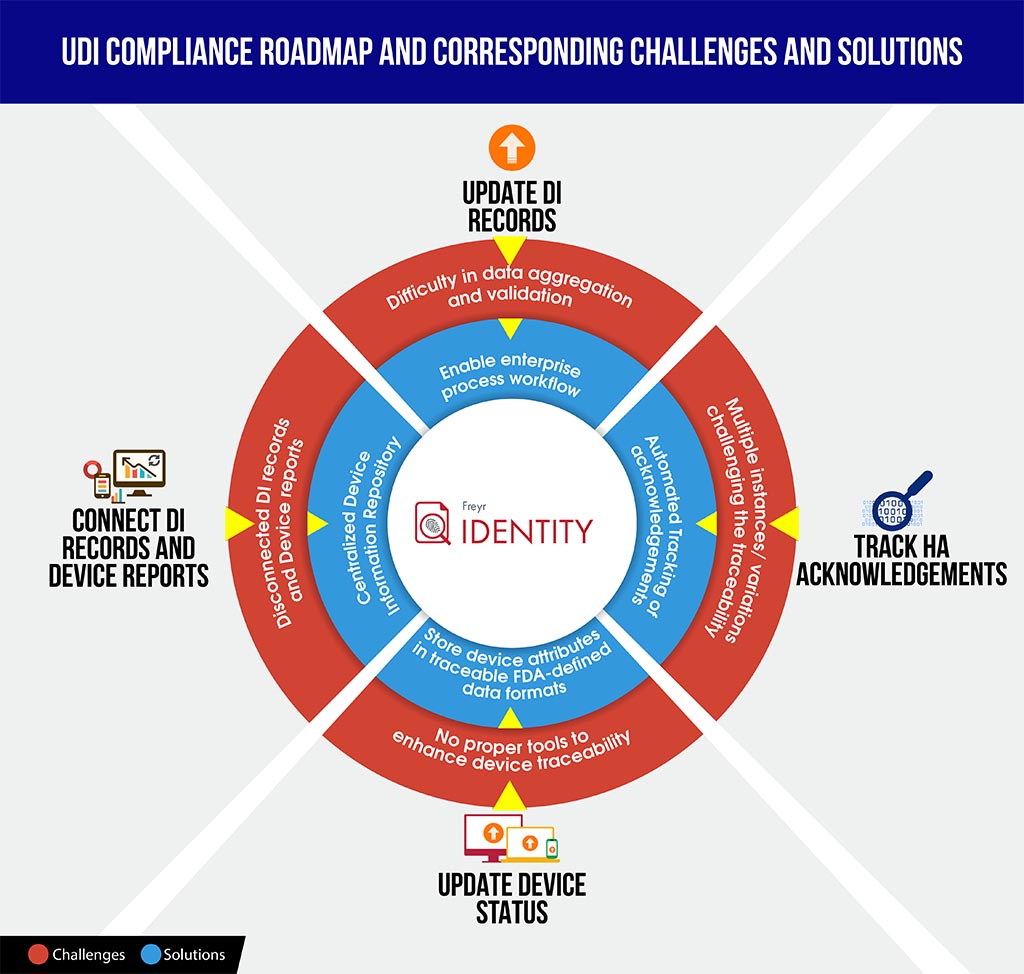
To smoothly navigate your organization through this complex compliance process, Freyr offers the best of both worlds – on-demand, fully configurable, UDI software solution, Freyr IDENTITY, as well as a UDI Centre of Excellence (CoE) that offers best in class, cost-effective and customizable UDI services built around your unique and demanding requirements.









