
For biocide product manufacturers, understanding the regulatory requirements of the United States can be a complex and time-consuming process. Biocidal products are essential for maintaining public health and safety, but before these products can be sold or distributed, they must comply with stringent rules established by the Environmental Protection Agency (EPA). Here's a step-by-step guide to help you through the registration process and ensure your products meet the necessary regulatory standards.
Overview of Biocide Regulation in the USA
In the United States, the EPA regulates biocidal products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). This law mandates that all biocidal products go through a comprehensive registration process. The goal is to ensure that each product is safe for humans, animals, and the environment, while also effectively meeting its intended purpose.
Biocidal Products Classification
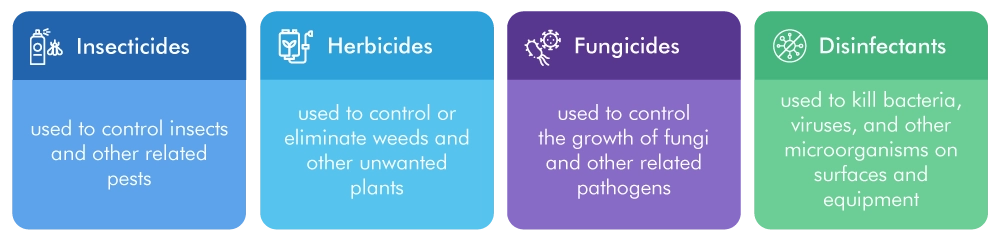
The EPA categorizes biocidal products into four main types:
Each of these categories requires different documentation, testing, and labeling to meet EPA guidelines.
Steps for Registering a Biocide Product in the USA
To successfully register a biocide or pesticide product with the EPA, applicants must submit a comprehensive application package containing the following key components:
- Cover Letter: While not mandatory, a cover letter is recommended. It should summarize the purpose of the submission and provide a list of all items included in the package.
- Application Form: Complete and submit the Application for Pesticide Registration/Amendment form (EPA Form 8570-1), signed and dated by the registrant or their designated agent.
- Product Identification: Provide clear identification details, including:
- Product name
- Trade name(s) (if applicable)
- Existing EPA Registration Number (if applicable)
- Draft Labeling: Submit draft labeling that complies with FIFRA requirements. This includes all labels and associated materials for the pesticide.
- Data Requirements: Provide data to demonstrate that the product will not cause unreasonable adverse effects. Required data may include:
- Residue chemistry
- Environmental fate
- Toxicology
- Reentry protection
- Spray drift analysis
- Impact on wildlife and aquatic organisms
- Plant protection efficacy
- Non-target organism effects
- Product performance
- Product chemistry information
Each of these categories may involve substantial documentation and studies to verify product safety and efficacy.
Following this step-by-step guide will help you efficiently understand the biocide product registration process, ensuring compliance with EPA regulations and a smoother path to market entry.
Key Considerations for Manufacturers
- Time Management: The registration process can be lengthy, often taking years. Starting early is essential to avoid delays.
- Supplier Collaboration: Work closely with your active substance suppliers to verify that they have the necessary Article 95 status.
- Compliance with Labeling Requirements: Labels must be easy to understand and offer clear guidance on the safe use of the product.
- Ongoing Product Evaluation: Even after receiving approval, your biocide product should be continuously assessed to ensure it remains safe and effective.
Conclusion
Biocide product registration in the USA is a highly regulated process that requires thorough preparation and ongoing attention to detail. By following these steps and key considerations, manufacturers can effectively complete the registration process and successfully bring their products to market.
For expert guidance on the biocide registration process, consult Freyr's team of specialists who can assist you in seamlessly bringing your biocide products to the US market while ensuring full regulatory compliance.









