
In the highly regulated life sciences industry, artwork preparation is a critical process that requires close collaboration between key stakeholders such as Quality Assurance (QA), Marketing, and Research & Development (R&D). Ensuring that artwork meets regulatory standards is not just about compliance; it directly impacts product launch timelines, market access, and patient safety. This blog will provide a detailed procedure for artwork preparation, highlighting the importance of cross-functional collaboration in achieving regulatory compliance and operational efficiency.
Step 1: Artwork Initiation and Content Development
- The marketing team defines the artwork requirements based on product specifications and target markets
- R&D provides technical information, such as product composition, dimensions, and packaging materials
- Regulatory affairs ensures that all content adheres to relevant guidelines and regulations
Step 2: Artwork Design and Creation
- Graphic designers create the initial artwork based on brand guidelines and regulatory requirements
- Marketing reviews the design for brand consistency and messaging
- R&D verifies the technical accuracy of the artwork, including dimensions, barcodes, and braille
Step 3: Artwork Review and Approval
- QA conducts a thorough review of the artwork for compliance with regulatory requirements and internal standards
- Marketing ensures that the artwork accurately represents the product and brand
- R&D validates the technical aspects of the artwork, such as dimensions and material specifications
- Cross-functional team meetings are held to discuss and resolve any issues or discrepancies
Step 4: Artwork Proofreading and Quality Control
- Quality Control Analysts (QCAs) perform a detailed proofreading of the artwork using electronic tools and manual checks
- QCAs ensure that all text, graphics, and technical components are accurate and comply with regulatory requirements
- Any errors or discrepancies identified are promptly addressed by the artwork operators before proceeding to the next stage
Step 5: Artwork Finalization and Approval
- The final artwork is submitted for regulatory approval, with all necessary documentation and supporting evidence
- Regulatory authorities review the artwork for compliance with applicable guidelines and standards
- Upon receiving regulatory approval, the artwork is considered finalized and ready for production
Step 6: Artwork Change Management
- Any changes to the approved artwork must go through a formal change control process
- QA, Marketing, and R&D collaborate to assess the impact of the proposed changes and ensure that they comply with regulatory requirements
- A revised artwork is created, reviewed, and approved following the same process as the initial artwork
By following this detailed procedure and fostering collaboration between QA, Marketing, and R&D, life sciences companies can ensure that their artwork preparation process is efficient, compliant, and aligned with their business objectives. Key benefits of this approach include:
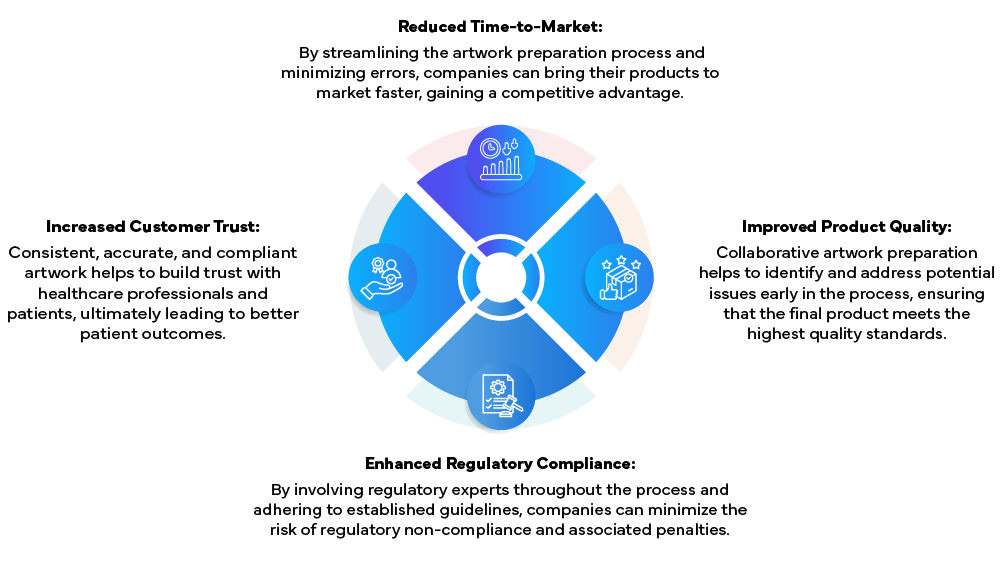
In conclusion, a well-defined and collaborative artwork preparation process is essential for life sciences companies looking to navigate the complexities of regulatory compliance while driving operational efficiency and market success. By fostering cross-functional collaboration and adhering to best practices, companies can ensure that their artwork preparation process is a strategic asset in their pursuit of regulatory compliance and market leadership.
Author: Nirupama Parate









