
On February 09, 2022, the European Medicines Agency (EMA) commenced the establishment of a Coordination Centre for Data Analysis and Real-World Integration Network (DARWIN EU®). It is the first step towards integrating Real-World Evidence (RWE) into assessing medicines across the European Union (EU). Ensuring the safety and efficacy of medicines is the prime activity of any Regulatory Agency, and RWE is proving to be a reliable source for gathering real-time evidence from global health care databases. Therefore, this move by the EMA is meant to enhance the quality of drugs in the EU region and make them available faster.
Major Activities Planned for the Coordination Centre for DARWIN EU®
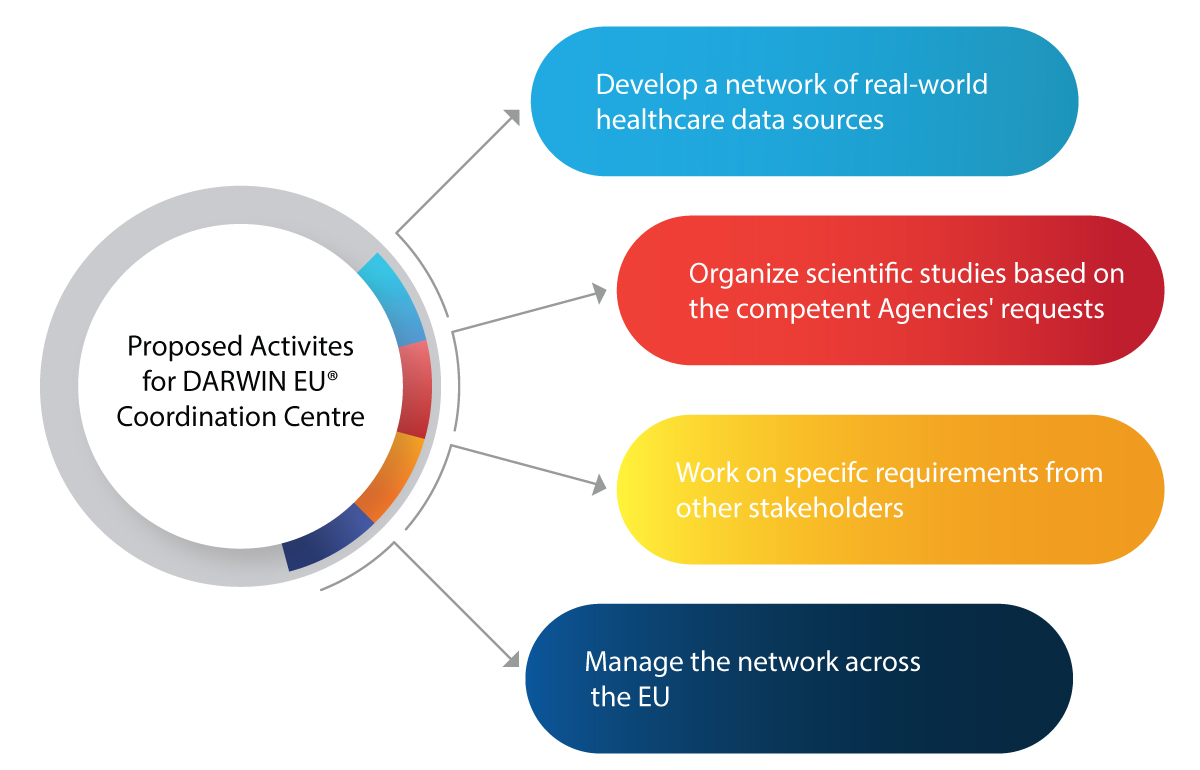
How is the DARWIN EU® Going to Help EMA and Relevant Authorities in the Regulatory Space?
Innovative drugs that are readily available to patients are the need of the hour. The Regulatory processes for the development, approval, marketing, and post-marketing surveillance take a substantial amount of time, making the demand for novel drugs unmet. Reducing the time-to-market while ensuring innovative drugs' safety, efficacy, and quality is the way forward. Several global Health Authorities are identifying the benefits of integrating RWE in their Regulatory approvals, and DARWIN EU® is the EMA’s initiative.
EMA plans to connect the European medicines Regulatory network to the European Health Data Space (EHDS) for better data exchange and enhanced access to various health data types under this program. It will also set standards for scientific evaluations and Regulatory decision-making.
Here are a few activities that the DARWIN EU® aims to achieve:
- Usage of RWE in decision-making activities for the regulation of new drugs, vaccines, etc.
- Integrating RWE in the entire lifecycle of a drug such as development, authorization, and post-market surveillance
- Prepare for any future medical emergencies such as pandemics
- Benefit the pharmaceutical industry with insightful and reliable RWE on patients, diseases, and usage of drugs
- Make informed Regulatory decisions based on RWE for the safe and effective use of medicines
- Improved accessibility of life-saving drugs to patients in need owing to accurate and timely data analysis
Proposed Timelines for DARWIN EU® to be a Fully Functional Network in the EU
The EMA also specifies the timelines for DARWIN EU® to be fully operational, and they look like:
- 2021 – Initiation of the project
- 2022 – Establishment of DARWIN EU®
- 2023 – Development of DARWIN EU® and defining its usages
- 2024 – Making DARWIN EU® fully operational
- 2025 – Enhance the Regulatory uses of health care data by increasing the scopes in terms of medicines, geography, etc.
The EMA is collaborating with the Erasmus University Medical Center Rotterdam for the establishment of DARWIN EU®. The goal of this partnership is the establishment of the Coordination Centre to create a distributed data network. Both of them have also taken up the tasks of conducting scientific studies, supporting Regulatory decision-making processes, and managing a catalog of real-world data sources.
What Does the Initiation of DARWIN EU® Mean for the Future?
The EMA and the Head of Medicines Agencies (HMA) have always worked together to identify the challenges, set time-bound goals, and define priorities in their five (05) year strategy documents. It is meant to provide strategic direction to the activities carried out by the European medicines Regulatory network.
The initiation of DARWIN EU® is a major step towards the delivery of the EMA’s Network Strategy. Conducting scientific studies to answer research questions, supporting the evaluation of medicines in the EU and maintaining metadata for medicine Regulatory activities are the main objectives. DARWIN EU® is foreseen as a major player in medicines regulation in Europe, and the pilot studies are proposed to be ready by the end of 2022.
If you are a drug manufacturer and wish to know more about RWE and how it is shaping the future of Regulatory decision-making, contact the Regulatory Affairs experts at Freyr.









