Pharmacovigilance (PV) involves activities for detecting, assessing, understanding, and preventing adverse drug effects/adverse events (AE) of drug products to ensure the safety of the patients. In the post-marketing phase of any pharmaceuticals or biologicals manufacturing company, budgets are mainly allocated to processes such as Adverse Drug Reaction (ADR) reporting and case processing, aggregate reporting, and signal detection. A leading England-based consulting firm survey shows that a PV department spends around 40-85% of the allocated budget on case processing operations. On the other hand, case volumes have been growing at a rate of 10-15% every year, as per the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS).
The digitalization era transformed PV systems and brought together PV, technology, quality, and Regulatory expertise across the industry to identify automation opportunities. Some key drivers of this paradigm shift are:
- Resource-intensive nature - Patient-focused strategies and treatment regimens have paved the way for increased PV processes. PV processes are currently resource-intensive, which makes them more prone to the risk of errors and operational inefficiencies
- Rising Adverse Events (AE)/ADR case volumes with increased disease burden - With novel diseases disrupting the health industry, in conjunction with the post-COVID19 surges, there has been an exponential increase in AE and ADR case volumes
- Stringent Regulatory demand - The norms from global health regulators have become more stringent, thus making organizations undertake a ‘digital move’ for managing PV operations.
Was the US FDA Already ‘Digitally Ready’ or is Upgrading for the Best?
The launch of the Food and Drug Administration Adverse Event Reporting System (FAERS) and Vaccine Adverse Event Reporting System (VAERS) helped the stakeholders to review the performance of their medicinal products in the market based on the cases of AE and ADRs. Under the project ‘Improving the Efficiency and Rigor of Pharmacovigilance at FDA,’ which started in 2014, researchers use the approaches of Natural Language Processing (NLP) and Machine Learning (ML) to analyze FAERS, and VAERS reports. The project aims to improve the performance and accuracy of databases used by the US FDA.
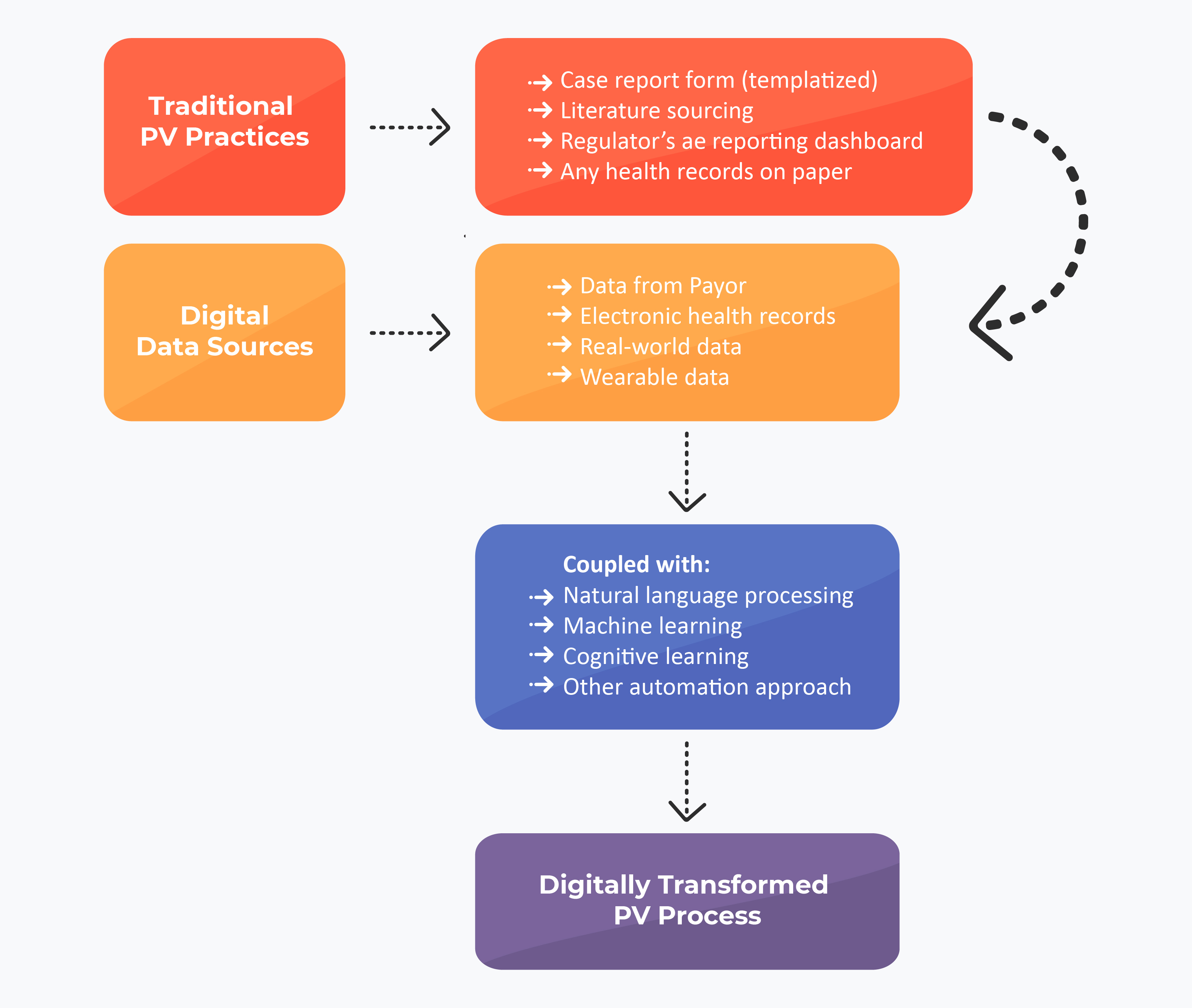
Digitalization in PV Operations
As many of the PV operations revolve around case processing, aggregate reporting, and signal detection, many digital PV operations propose an ‘Overlap approach.’ This approach mainly works on the machine learning (ML) model and drives the data on the information which is collected, analyzed, and processed in either of the three operations. The second approach is phase-wise learning and experimenting with various tools until bringing to function for operations.
ML Approach for Future PV Operations
One of the systematic reviews published in early 2022 indicated the potential use of NLP in overcoming conventional PV practices. The data scientists revealed that the NLP could map and identify different types of AEs and relevant terms for the selected literature. With such complementing and reliable results, digital PV operations would be the future across various PV-based organizations.
The power of technology needs to be harnessed to transform the pharmacovigilance industry, enabling it to focus more on analysis and prediction to allow agile decision-making, maximization of benefit/risk for patients and healthcare providers, and increased efficiency of healthcare. Pharmacovigilance business imperatives can now be easy-going when partnered with an experienced and knowledgeable partner like Freyr. Moreover, a partner like Freyr can cater to customized business queries and resolve them with a quick turnaround time. Consult Freyr!