
Introduction
The landscape of drug discovery and development is experiencing a profound transformation, driven by the emergence of precision medicine. In this evolving paradigm, Quantitative Structure-Activity Relationship (QSAR) modeling emerges as a potent tool that has the potential to revolutionize the pharmaceutical industry. It is no longer just about discovering drugs; it is about crafting treatments as unique as the individuals they serve. In this blog, we detail how QSAR is changing the drug discovery game, shifting the focus from molecules to patients and ushering in an era of personalized therapeutics.
Understanding the Need for QSAR and Precision Medicines’ Synergy
Precision medicine deals with the treatment effect at the genomic and molecular biology level. It has redefined the healthcare approach. Patient individuality and tailored medical interventions are the features of precision medicine leading to the treatment of various new emerging and lifestyle-borne diseases.
Owing to the benefits of QSAR and the data repository available today, from genomics to clinical records, this synergy between precision medicines and QSAR can bridge the gaps between molecular structures and patient-specific drug responses.
QSAR's Role and Application with Precision Medicine
QSAR modeling is not new, but its relevance is growing exponentially in the era of precision medicine. It offers a quantitative, data-driven approach to understanding the relationship between molecular structures and biological activity. With precise patient data, scientists can now create QSAR models that predict how an individual's body will respond to a particular drug or treatment. This is where personalization begins – the ability to predict efficacy, dosage, and potential side effects for a specific patient.
Factors aligning to personalized medicine are not limited to genetic variations. Moreover, it also encompasses a patient's lifestyle, environment, and microbiome. QSAR, when integrated with diverse data sources, can provide a holistic view of what makes a treatment personalized.
Challenges and Ways to Overcome Them
The amalgamation of QSAR with precision medicine is a promising endeavor, but overcoming these challenges is pivotal for the seamless integration of personalized drug design into healthcare practices.
- Data Quality and Quantity: Ensuring the data is vast, accurate, and reliable is a monumental challenge. Data must be collected, stored, and shared securely while respecting patient privacy and adhering to evolving data protection regulations.
Collaboration between healthcare institutions, researchers, and technology companies is critical in addressing this challenge. Data-sharing protocols and standards must be developed to harmonize information from diverse sources.
- Regulatory Alignment: The Regulatory landscape has traditionally been structured around mass-produced drugs intended for a broad patient population. Precision medicine challenges these established frameworks, as treatments are increasingly tailored to individual patients. Regulatory bodies worldwide are grappling with how to adapt to these new paradigms.
In response, regulators are working closely with the pharmaceutical industry to create pathways to approve personalized drugs. This entails a shift from a one-size-fits-all approach to a more flexible Regulatory model that evaluates drugs on a case-by-case basis. Clear guidelines, collaborative discussions, and agile adaptation of regulations are necessary to ensure innovative treatments can reach patients in need.
- Managing the Vast Amount of Patient Information: Precision medicine generates an unprecedented volume of patient information, from genomic data to lifestyle records. This wealth of data presents and demands advanced data management and analysis capabilities.
Artificial Intelligence (AI) and Machine Learning (ML) are employed increasingly to sift through vast datasets, identify relevant patterns, and assist in the development of QSAR models. These technologies can rapidly process and interpret complex patient data, facilitating more accurate predictions for personalized treatments.
- Ethical and Privacy Concerns: Precision medicine and QSAR raise ethical questions concerning data ownership, consent, and potential misuse. Patients must have agency over their genetic and medical data and ensuring that their rights and privacy are respected is paramount.
Role of Regulatory Vendors
In the era of precision medicine and the integration of QSAR modeling, Regulatory vendors and medical writers play pivotal roles in bridging the gap between scientific innovation and Regulatory compliance.
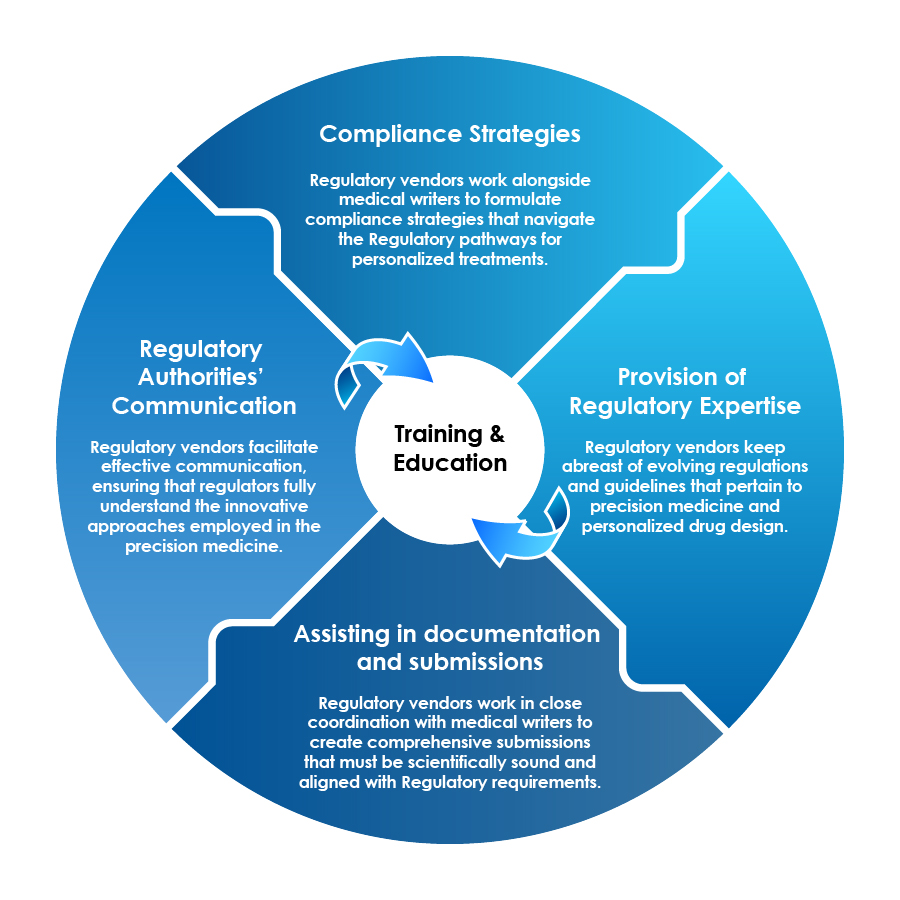
Training and Education is a Pivotal Contribution by Regulatory Vendors toward Their Stakeholders
Conclusion
The convergence of QSAR and precision medicine is rewriting the rules of drug discovery. It is a transformation that moves us from generic treatments to those tailored for individuals. As we look to the future, we envision a healthcare landscape where patients are not statistics but unique individuals with personalized treatment plans. QSAR, as a powerful tool, is steering us towards this future. With each breakthrough, we draw closer to an era where healthcare is as unique as the people it serves, revolutionizing the way we approach medicine. With the right Regulatory vendor at your disposal, the roadmap toward a successful submission basis such as precision medicine is now an easy-going task. Consult us to learn more about our expertise!









