
In the increasingly complex world of clinical research, pharmaceutical and biotech companies often rely on external partners to conduct clinical studies. These partnerships provide significant benefits, including access to specialized expertise, advanced technologies, and enhanced operational efficiency. However, to maximize these benefits and ensure the success of clinical studies, it is crucial to maintain robust Applicant/ Sponsors oversight. This blog explores the importance of Applicant/ Sponsors oversight in contracted clinical studies and how it can lead to more reliable and impactful research outcomes.
The Role of Applicant/Sponsors Oversight:
Applicant/Sponsors oversight refers to the proactive management and supervision of contracted clinical studies by the sponsor company.
What does Applicant/ Sponsors oversight involve?
Effective oversight ensures that the study is conducted according to predefined standards and regulations, thereby safeguarding the safety, integrity and validity of the research findings.
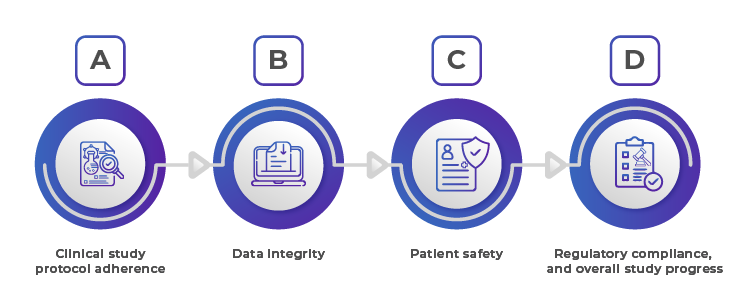
Key Aspects of Applicant/ Sponsors Oversight:
1. Clinical study Protocol Adherence
Ensuring that the contracted organization follows the study protocol to the letter is critical. Any deviations can compromise the validity of the study results and lead to regulatory scrutiny. Regular audits and monitoring visits are essential to verify adherence to the protocol. The sponsor must provide clear, detailed guidelines and conduct periodic training to ensure all stakeholders understand and adhere to the protocol.
- Detailed Protocol Training: Providing comprehensive training on the study protocol to all personnel involved in the clinical study ensures that everyone understands the study's objectives, procedures, and expected outcomes. This includes creating detailed training manuals and conducting regular refresher sessions.
- Audit Trails: Establishing audit trails helps track every change made to the study protocol and data. This documentation is crucial for maintaining transparency and accountability, enabling the identification of any deviations from the protocol.
2. Data Integrity
Accurate and reliable data is the cornerstone of any clinical study. Oversight mechanisms should include regular data reviews, source data verification, and audits to ensure that data is collected, recorded, and reported accurately. This also involves ensuring that electronic data capture systems are validated and secure. Implementing robust data management practices and using advanced data analytics can help in the early detection of discrepancies and anomalies.
- Data Management Systems: Employing sophisticated data management systems that support real-time data capture, validation, and analysis ensures the integrity and accuracy of clinical data. These systems should be compliant with regulatory standards such as 21 CFR Part 11.
- Data Quality Checks: Regular quality checks and data cleaning processes help identify and rectify any inconsistencies or errors in the data, ensuring that the final dataset is reliable and ready for analysis.
3. Patient Safety
The safety of study participants is paramount. Sponsors must ensure that the contracted organization has robust systems for monitoring adverse events and promptly implementing corrective actions. This includes regular safety reviews and ensuring compliance with Good Clinical Practice (GCP) guidelines. Regular safety audits, patient feedback mechanisms, and real-time monitoring tools are essential to safeguard patient welfare.
- Adverse Event Reporting Systems: Implementing efficient adverse event reporting systems ensures that potential safety issues are identified and addressed swiftly. These systems should allow for immediate reporting and tracking of adverse events.
- Patient Monitoring Technologies: Advanced patient monitoring technologies, such as wearable devices, can provide continuous health data, enabling timely intervention and ensuring patient safety throughout the study.
4. Regulatory Compliance
Clinical studies must adhere to local and international regulatory requirements. Effective oversight involves ensuring that the contracted organization complies with these regulations and that all necessary approvals and documentation are in place. This includes understanding the regulatory landscape of each region where the study is conducted and ensuring all documentation is updated and readily available for regulatory reviews.
- Regulatory Training: Providing ongoing training on regulatory requirements to all study personnel ensures that they are aware of and comply with all relevant regulations and guidelines.
- Regulatory Documentation Management: Implementing robust systems for managing regulatory documentation ensures that all required approvals, reports, and records are maintained accurately and are easily accessible for audits and inspections.
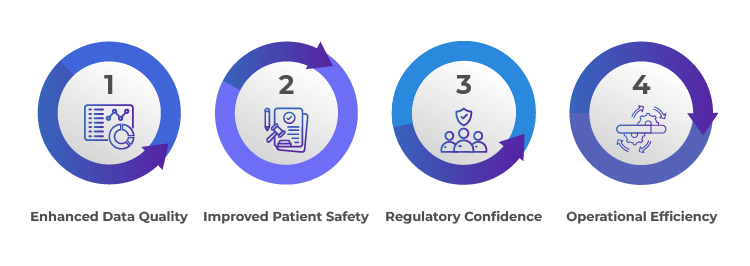
Benefits of Effective Applicant/ Sponsors Oversight
Summary
Applicant/ Sponsors oversight in contracted clinical studies is not just a regulatory requirement but a strategic imperative. It ensures the integrity, safety, and reliability of clinical research, ultimately leading to more robust and credible study outcomes. A regulatory partner’s expertise in regulatory services and comprehensive oversight capabilities can help you navigate the complexities of clinical studies, ensuring that your research is conducted to the highest standards. Partner with Freyr to enhance your clinical research oversight and achieve your study goals with confidence.









