
Regulatory writing plays a crucial role in the pharmaceutical and healthcare industries, bridging complex scientific information and various stakeholders. The art of tailoring Regulatory writing to different audiences is essential for effective communication and compliance. This blog will explore how to adapt Regulatory writing for three (3) key audiences: patients, clinicians, and regulators.
Understanding the Importance of Audience-Specific Writing
Regulatory writing encompasses various documents, each serving a specific purpose and targeting different readers. The ability to tailor content to these diverse audiences is critical for ensuring that information is accurate but also accessible and actionable.
Primary Pointers considering the tailoring approach:
Writing for Patients: Simplifying Complex Information
When crafting Regulatory documents for patients, the primary goal is to convey essential information clearly and understandably without sacrificing accuracy.
- Use of Plain Language
Employ everyday words and avoid technical jargon. When medical terms are necessary, provide clear explanations.
- Visual Aids and Formatting
Incorporate diagrams, charts, and infographics to illustrate complex concepts. Use bullet points, headings, and short paragraphs to enhance readability.
- Addressing Common Concerns
Anticipate and address potential questions or concerns that patients might have about the medication or treatment.
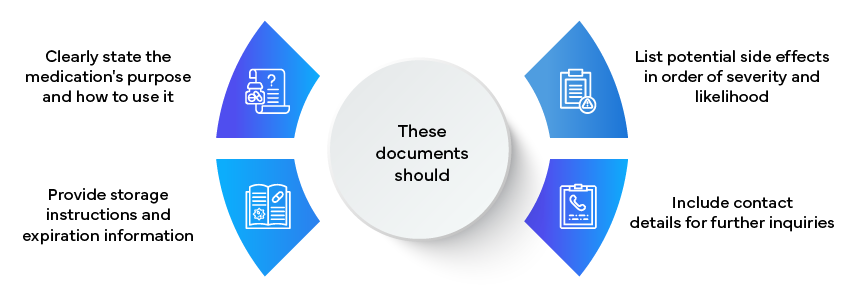
Example: Patient Information Leaflets
These documents should:
Writing for Clinicians: Balancing Detail and Practicality
Clinicians require comprehensive yet concise information to make informed decisions about patient care. Regulatory writing for this audience must balance scientific detail and practical applicability.
- Emphasis on Clinical Relevance
Focus on information impacting patient care, such as dosing guidelines, contraindications, and drug interactions.
- Structured Presentation of Data
Organize clinical trial results and safety data in a logical, easy-to-navigate format. Use tables and graphs to summarize key findings.
- Inclusion of Evidence-Based Recommendations
Provide clear, evidence-based guidance on the appropriate drug or medical device use in various clinical scenarios.
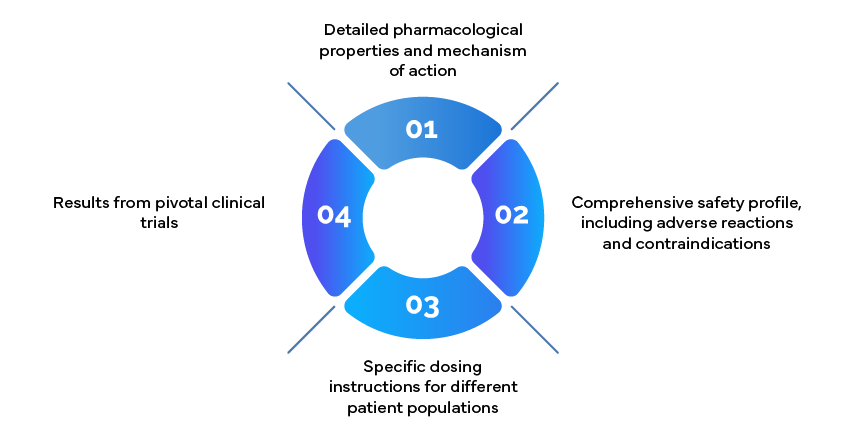
Example: Prescribing Information
Prescribing information documents, also known as package inserts, are crucial resources for clinicians. These should include:
Writing for Regulators: Precision and Compliance
Regulatory agencies require exhaustive, scientifically rigorous documentation to evaluate the safety and efficacy of new products. Writing for this audience demands meticulous attention to detail and strict adherence to Regulatory guidelines.
- Adherence to Regulatory Guidelines
Ensure that all documents comply with the specific format and content requirements of Regulatory bodies such as the FDA or EMA.
- Comprehensive Data Presentation
Provide a thorough analysis of all relevant data, including detailed descriptions of study methodologies, statistical analyses, and results interpretation.
- Transparency in Reporting
Disclose any limitations or potential biases in the research and address how these factors may impact the conclusions.
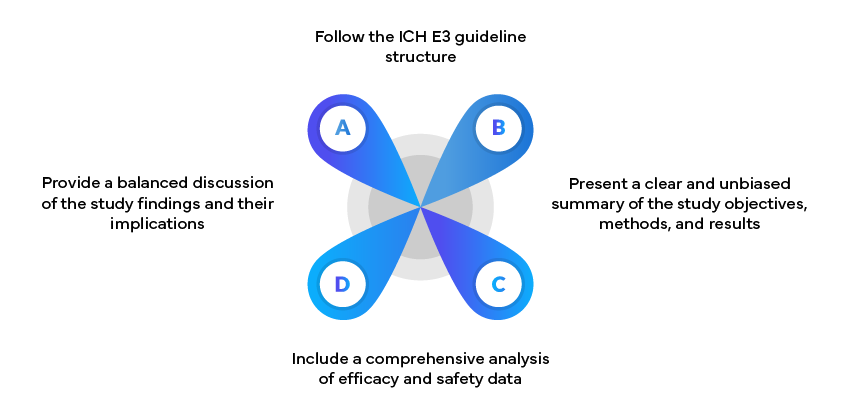
Example: Clinical Study Reports
Clinical Study Reports (CSRs) are critical documents in Regulatory submissions. These should:
Conclusion
Tailoring Regulatory writing for different audiences is a complex but essential task in the pharmaceutical and healthcare industries. By adapting content and presentation styles to meet the specific needs of patients, clinicians, and regulators, writers can ensure that critical information is effectively communicated and understood.
The ability to craft clear, accurate, and audience-appropriate Regulatory documents is not just a matter of compliance; it directly impacts patient safety, clinical decision-making, and the successful development and marketing of new healthcare products. As the Regulatory landscape continues to evolve, the importance of skilled Regulatory writing that can bridge the gap between complex scientific data and diverse stakeholder needs will only grow. Regulatory writers play a crucial role in advancing public health and fostering innovation in the medical field by focusing on patient clarity, clinical relevance for healthcare professionals, and comprehensive precision for regulators. Mastering the art of audience-specific Regulatory writing is thus an invaluable skill in today's rapidly evolving healthcare environment.









