
A new report from a European generics trade association says that extreme cost-containment policies can adversely affect the generics supply chain vide consolidation of generics drug production and withdrawal of such products from the market. A recent press release by the Medicines for Europe further states that there is evidence that extreme cost-containment policies are producing counterproductive effects on generic medicines. The European Union (EU) market is also facing a withdrawal risk for important and less expensive medicines owing to the same.
Dated November 23, 2021, the report draws references from the European Commission report from 2018, which offers patent protection up to thirteen (13) years for innovator drugs. Supplementary protection certificates for Active Pharmaceutical Ingredient (API) for five (05) more years make an extended total of up to twenty-five (25) years of API protection in the EU market. During this period, the price of the product remains the same, and it is saved from competitor products.
Manufacturers who prefer off-patent innovation find it to be a cost-effective alternative. Though the total cost of off-patent products is about/more than half of that when developing a de novo pharmaceutical (a drug design involving an iterative process in which the three-dimensional structure of the receptor is used to design novel molecules), it is coming off as a better option.
Most drug manufacturers are finding drug repurposing to be the ideal option in off-patent innovation. This is a process in which existing drugs are investigated for identifying new therapeutic uses. Also known as drug repositioning, several pharmaceutical companies are developing new drugs using this efficient, time-saving, and cost-effective strategy.
The Commission report of 2018 contains the protocol to be followed for drug repositioning. It also includes the Regulatory incentives that are available to Marketing Authorization Holders (MAHs) of certain pharmaceuticals. According to the latest report from the aforementioned trade association, the following four (04) benefits should be offered at different stages of the repurposing/repositioning process.
Proposed Incentives for Sponsors at Varied Stages of the Repositioning Process
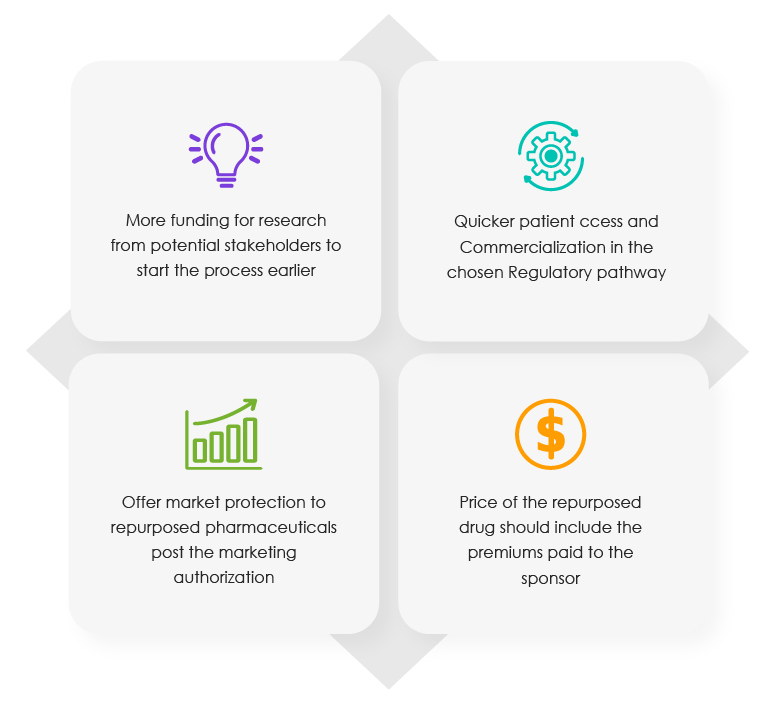
To encourage off-patent innovation in the EU market, much work has been carried out in the recent past. This includes building a public-private framework for drug repurposing by a team of experts. The current report bases its findings on this framework that was launched in October 2021. It aims to make the most of the untapped source of innovation in the pharmaceutical space to match the unmet demand for life-saving drugs.
If you are a generics manufacturer looking for EU market entry, timely information on the latest Regulatory updates and consultation with a partner like Freyr can make you practice the best of compliance procedures and save you a lot of cost. Stay informed. Stay compliant.









