
The United States Food and Drug Administration (US FDA) released guidance in December 2022, a revision of the guidance titled ‘Failure to Response to an ANDA Complete Response Letter (CRL) Within the Regulatory Timeframe Guidance for Industry,’ of July 2022. It is meant to guide the applicants of Abbreviated New Drug Applications (ANDAs) submitted under section 505(j) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 355(j)). A complete and relevant response to a CRL is crucial for the approval of the generic drug. The guidance document offers recommendations regarding the repercussions of receiving a CRL and the actions FDA may take if the CRL is not responded to within the specified timeframe.
The FDA must ensure that the approved generic drugs meet the safety, quality, effectiveness, and affordability standards. If the ANDA does not meet the FDA Regulatory requirements, a CRL is sent to the applicant, and it includes the deficiencies identified during its assessment.
Following is a figurative description of what an applicant can do within a year of receiving the CRL:
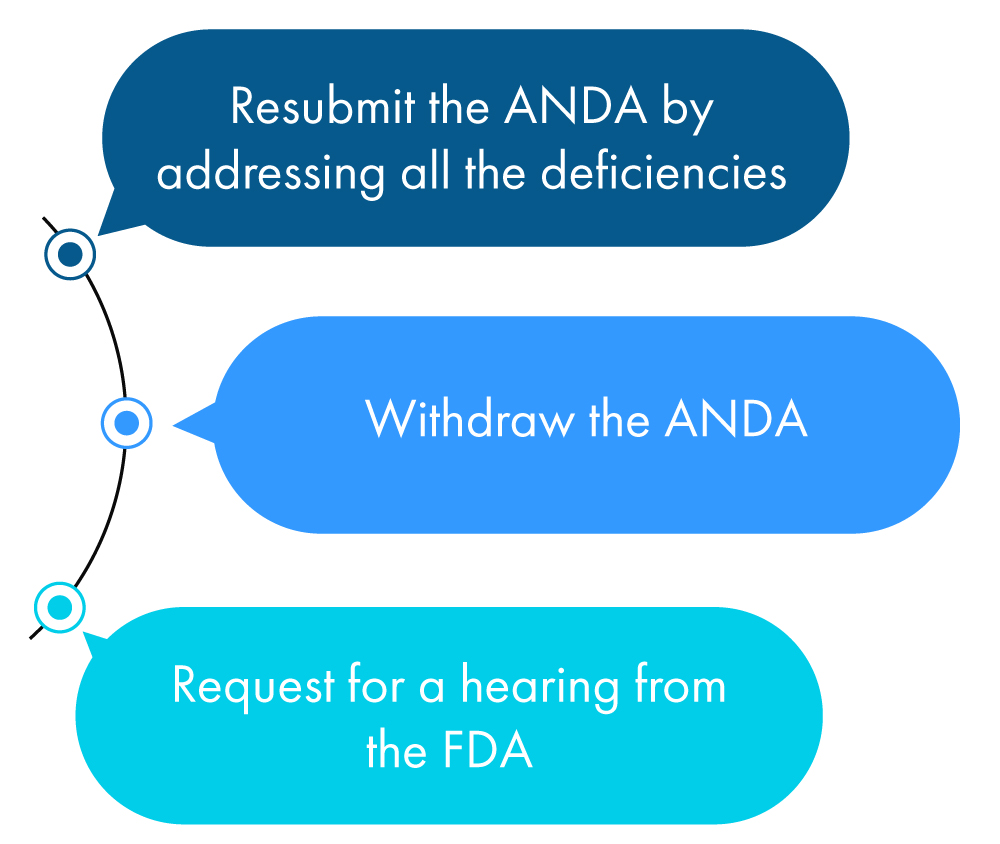
If none of the above actions are taken by the applicant, the FDA considers this as a request to withdraw the ANDA unless the applicant has requested an extension of time to check all the discrepancies mentioned in the CRL.
What Happens After One (01) Year of Issuing the CRL?
The FDA sends a written notification that the applicant has thirty (30) days from the notification for the following:
- Give reasons as to why the ANDA should not be withdrawn
- Request for an extension of time to deal with all the deficiencies mentioned in the CRL
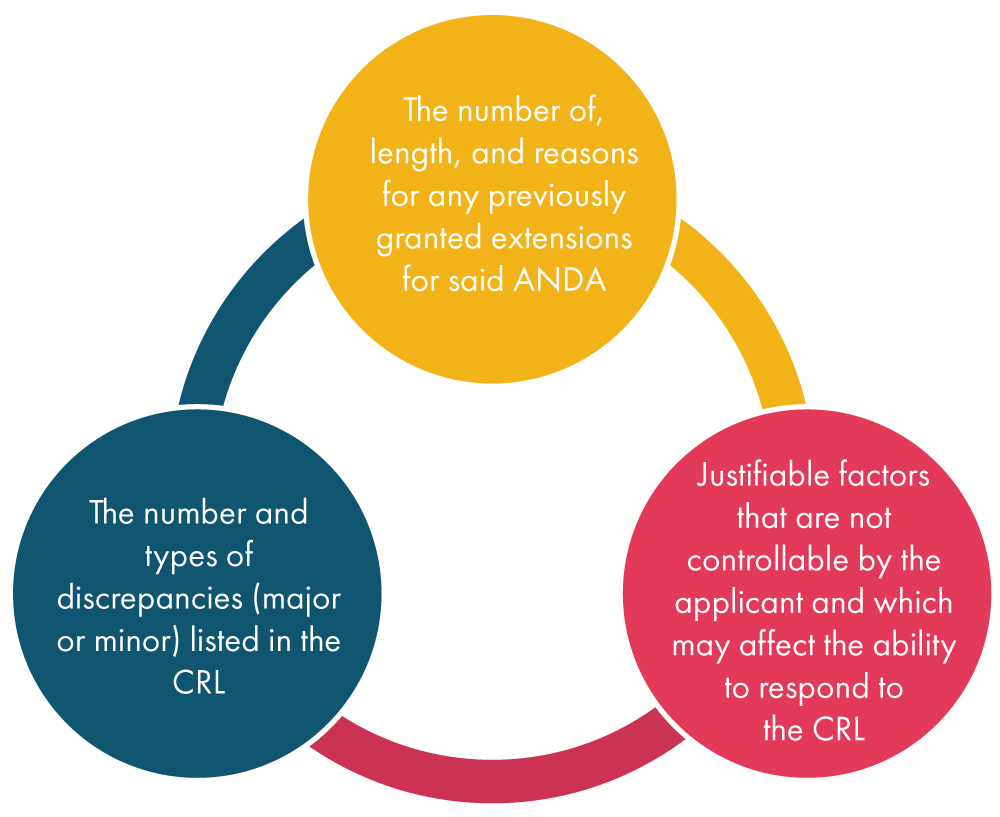
Once the FDA receives a request for an extension from the applicant, it considers various factors as mentioned below and subsequently decides on the request:
What is an Amendment?
The applicant needs to submit a request for an extension to respond to a CRL through an amendment. The FDA classifies an amendment as a major or minor one based on the effect it has on the approval process.
Vital FDA Recommendations the Applicants Should be Aware of:
- The FDA will reclassify a minor amendment as a major one if the amendment is submitted after a year of the CRL, unless the ANDA is for a product on the drug shortage list under section 506E of the FD&C Act (21 U.S.C. 356e), or is the subject of a response to a Public Health Emergency as declared by the Secretary of the US Department of Health and Human Services under section 319 of the Public Health Service Act (42 U.S.C. 247d), or is anticipated to be subject to the same criteria as apply to such a declaration, at the time of submission.
- If the applicant fails to address the discrepancies in the CRL within the extended period granted by the FDA, the latter may consider withdrawing the ANDA.
- If the applicant needs more than the thirty (30)-days extension period, he/she can request a further extension. The amendment then needs to include new information that will allow the FDA to determine whether the extension can be granted or not.
Getting a CRL from the FDA can be quite daunting for ANDA applicants. From addressing the discrepancies to citing the correct reasons/information for an extension, all relevant requirements should be met to ensure faster drug approval. Freyr is a proven Regulatory expert with extensive knowledge in responding to CRLs and ensuring compliance with FDA requirements. Contact us now for end-to-end strategic support in ANDA submissions.









