
The Clinical Trials Information System (CTIS) is a centralized database that supports the conduct of clinical trials in the European Union (EU). It was launched in January 2022 and is a key component of the Clinical Trials Regulation (CTR), which aims to improve the efficiency and transparency of clinical trials in the EU. The CTIS has a number of features that make it a valuable tool for clinical trial sponsors, researchers, and Regulatory authorities.
These features include:
- A single point of entry for submitting and managing clinical trials in multiple countries.
- A secure and efficient way to exchange information between sponsors, researchers, and regulators.
- A searchable database of clinical trials that is accessible to the public.
- Tools to support the monitoring and oversight of clinical trials.
- CTIS is already having a positive impact on the landscape of clinical trials in the EU. It makes it easier and faster to conduct clinical trials and ensures that clinical trial data is more transparent and accessible.
Explore CTIS Solutions for Your Clinical Trials
Optimize Your Clinical Trials Now
How CTIS is changing the landscape of clinical trials:
- CTIS is making it easier to conduct clinical trials in multiple countries:
Previously, sponsors had to submit separate applications to each country in which they wanted to conduct a clinical trial. This was a time-consuming and expensive process. CTIS allows sponsors to submit a single application that is valid in all EU Member States. This made it much easier and faster for sponsors to conduct clinical trials in multiple countries.
- CTIS is improving the efficiency of the clinical trial review process:
Regulators in different countries can now collaborate more easily through CTIS. This means can now be reviewed quicker and more efficiently.
- CTIS is making clinical trial data more transparent:
The public website of CTIS provides access to information about all clinical trials that are registered in the system. This information includes the trial protocol, the results of the trial, and any safety concerns. This increased transparency is helping build public trust in clinical trials.
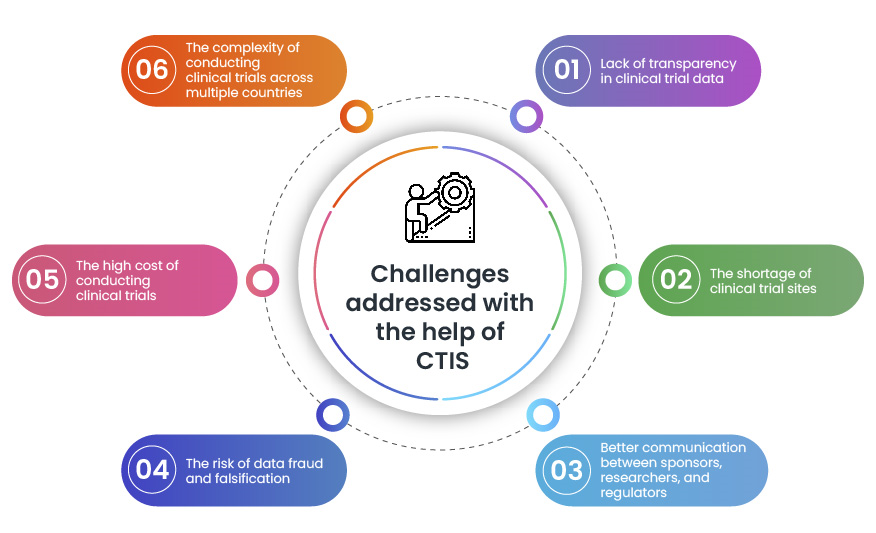
CTIS is a valuable tool that helps in addressing some key challenges faced during clinical trials. By making it easier, faster, and more transparent to conduct clinical trials, CTIS enables bringing new treatments to patients quickly.
CTIS is still a relatively new system, but it has the potential to revolutionize the way clinical trials are conducted in the EU. In a world where scientific advancements are more vital than ever, CTIS, in synergy with a global Regulatory partner like Freyr, holds the key to unlocking new frontiers in healthcare. As we embrace these transformative technologies, we pave the way for faster, safer, and more impactful discoveries that have the potential to shape the future of medicine for generations to come.









