
The digital age has transformed the pharmaceutical industry, with the FDA embracing electronic submissions for streamlined drug development. A crucial element of this shift is the use of Single Structure-Data Files (SD files) within the Electronic Common Technical Document (eCTD) format.
This blog post delves into the world of SD file preparation for FDA submissions, equipping you with the knowledge to assist you with efficient and compliant submissions.
Why are SD Files Important for the FDA?
SD files encapsulate vital chemical structure information for new drugs. They are mandatory for submissions like New Drug Applications (NDAs), Abbreviated New Drug Applications (ANDAs), and Drug Master Files (DMFs) under the FDA's Knowledge-Assisted Structure-Activity Relationship (KASA) program.
Here's why SD files are crucial:
- Enhanced Efficiency: SD files eliminate the need for manual structure drawings, allowing for automated chemical registration, analysis, and structure extraction. This translates to faster review times for the FDA.
- Improved Accuracy: The standardized format of SD files minimizes errors associated with manual data entry, ensuring the integrity of the submitted information.
- Streamlined Review Process: SD files facilitate easier manipulation and visualization of chemical structures by reviewers, leading to a more efficient review process.
While not mandatory for all Chemistry, Manufacturing, and Controls (CMC) data, including SD files in submissions, is highly recommended. Their benefits contribute significantly to a smoother and more expedited drug approval process.
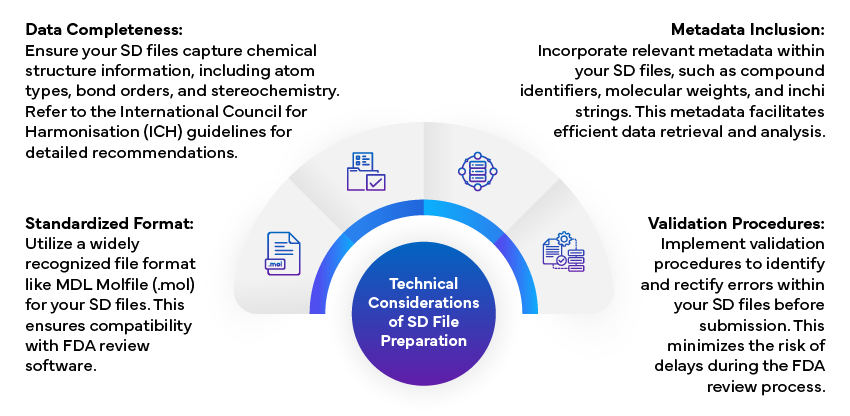
Technical Considerations of SD File Preparation
Preparing compliant SD files requires thorough attention to detail and adherence to technical guidelines.
The Role of Regulatory Submissions Experts
While SD file preparation might seem straightforward, ensuring compliance and technical accuracy can be complex. This is where regulatory submission experts from specialized firms become invaluable. These experts possess in-depth knowledge of FDA regulations, ICH guidelines, and the intricacies of SD file formats. They can provide you with:
- Guidance and Expertise: Submissions experts offer comprehensive guidance on SD file preparation, ensuring your files meet all regulatory requirements.
- Data Validation and Review: They thoroughly validate your SD files, identifying and rectifying any errors before submission.
- Streamlined Submission Process: Their expertise streamlines the entire submission process, saving you valuable time and resources.
Conclusion
SD files play a critical role in facilitating efficient and accurate FDA submissions for new drugs. By understanding the importance of SD files, adhering to technical considerations, and leveraging the expertise of regulatory submissions specialists, you can ensure your filings meet all regulatory requirements and pave the way for successful drug approval. Trust a Global Regulatory leader to enhance your SD preparations and accelerate your submissions to the HA.
Author: Sonal Gadekar









