
The regulatory writing and publishing segment claimed a dominant 36.6% revenue share in 2022 (GVR report), propelled by outsourcing in the biopharmaceutical and medical device industries. Staying ahead in the fast-paced world of regulatory submissions is paramount. Embracing game-changing technologies and keeping updated yields faster approvals, fewer errors, and heightened Regulatory compliance. Explore how an updated submission software revolutionizes the regulatory landscape, unlocking untapped potential and driving unprecedented success. Also stay tuned for an exclusive solution that will reshape the way you manage your regulatory submissions towards the end of this blog!
Streamlining Workflows: Leave behind manual processes and paperwork. Updated submission software brings cutting-edge features that streamline workflows, making regulatory navigation effortless. With automated document management and seamless collaboration, efficiency is optimized, and errors are reduced, saving valuable time and resources.
Enhancing Regulatory Compliance: Trust and credibility are built on compliance. Updated submission software seamlessly incorporates Regulatory compliance into the process. Advanced features like version control, audit trails, and real-time regulatory updates ensure adherence to ever-changing regulations. By mitigating risks and avoiding penalties, organizations safeguard their reputation and stay competitive.
Driving Innovation: Fuel innovation with the right submission software. Stay ahead by leveraging cutting-edge technologies and best practices.
Transforming User Experience: User-centric design and intuitive interfaces make complex regulations accessible. Personalized dashboards and robust training resources empower users to maximize software potential. Simplified navigation enhances the user experience, increasing productivity and ensuring a smooth transition.
Achieving Unparalleled Success: Unlock unparalleled success with updated submission software. Streamlined workflows, enhanced Regulatory compliance, innovation, and improved user experience pave the way. Surpass goals, gain a competitive advantage, and lead in your industry with the right tools and strategies.
The Regulatory Submission Process
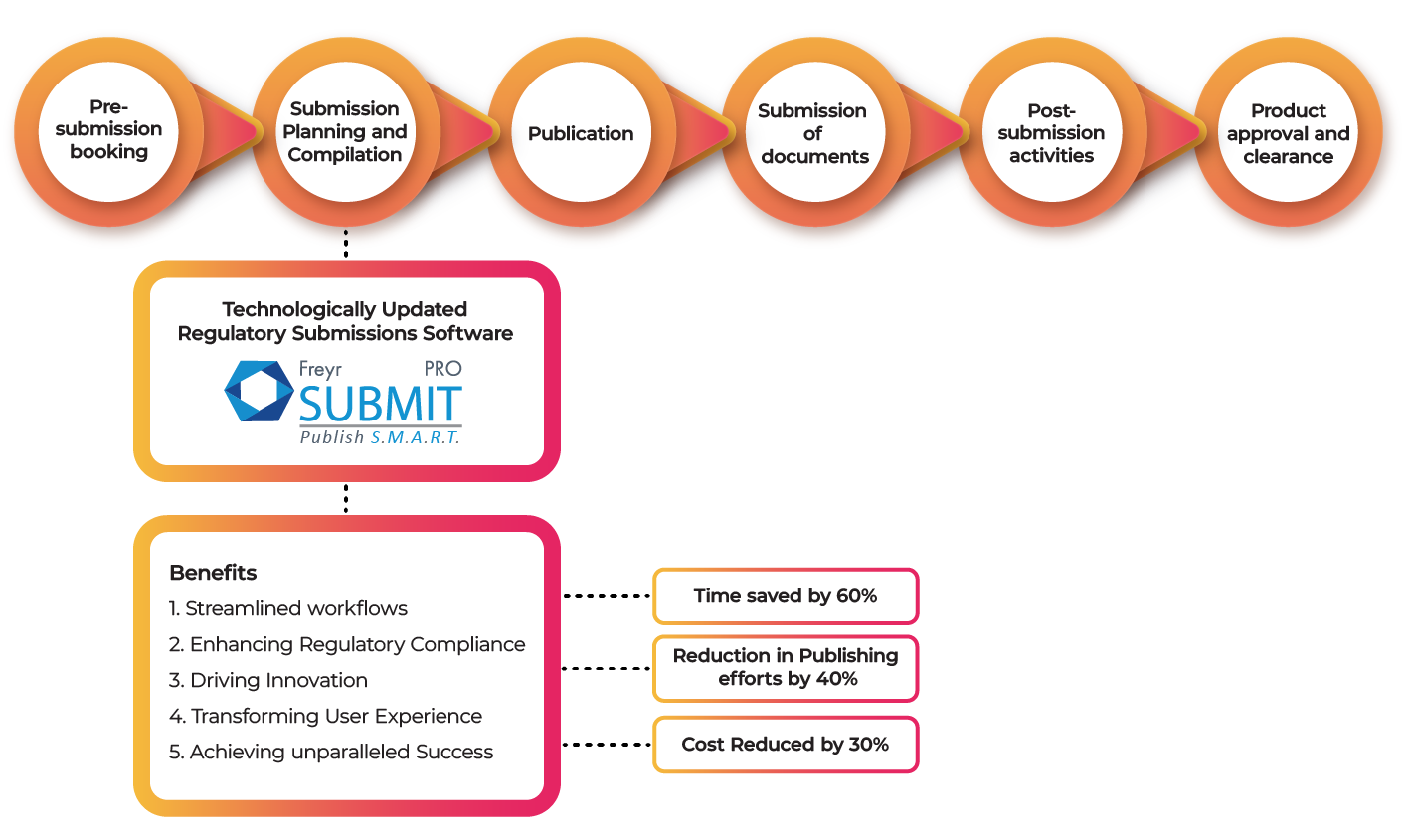
Elevate your regulatory submissions with updated software, ensuring Regulatory compliance and seamless management. Collaborate effortlessly on a centralized platform, where stakeholders review and approve submissions with ease. Embrace the future and unlock new possibilities with Freyr Digital, a pioneer of innovative tech-enabled products like Freyr SUBMIT PRO. Our cutting-edge technology, 21 CFR Part 11 standards, and user-friendly approach make us the go-to software for compliant eCTD submissions across multiple health authorities.
Stay ahead of the curve with regular updates and technological advancements. Exciting news! The latest version of Freyr SUBMIT PRO is now available. It has now got support to more ASEAN countries as well as support China eCTD Submissions. Partner with us to streamline your regulatory operations, boost productivity, and elevate submission quality. Request a demo today and experience the perfect blend of expertise and tools. Propel your organization from good to great.









