
Medicine labels act as a first-hand guide for the users and help to use the products qualitatively. Therefore, it is highly important to that the information available on the labels is consistent, easy-to-read and error-free, and on par with the global standards. In order to align with the same, the Therapeutic Goods Administration (TGA) has introduced a few changes in the way the labeling information is presented on the products. The purpose of introducing these changes is to improve the clarity and consistency of information provided to the healthcare professionals.
What Are The New Label Changes?
Though the new information is already taken into consideration and implemented for manufacturers, it is wise to know them in detail for a comprehensive presentation. Below listed are a few of them, that the manufacturers must work on, when defining their medicinal labels.
1. Active Ingredient Information
According to the new rule, Active Ingredients should be:
- Placed more obviously on the front side of the medicine pack
- Should be placed below or next to the name of the medicine on the front of the medicine pack
- Must be easily comparable between the medicines
2. Medicine Information
To ensure that the users and healthcare professionals make an informed decision, the medicine information should be clearer in terms of:
- Critical health information in distinctive labels
- All the mandatory information must be presented in a clearer and more recognizable way against the background color to improve its legibility
- Declarable substances, such as allergens, are required to be mentioned on the label
3. Critical Health Information
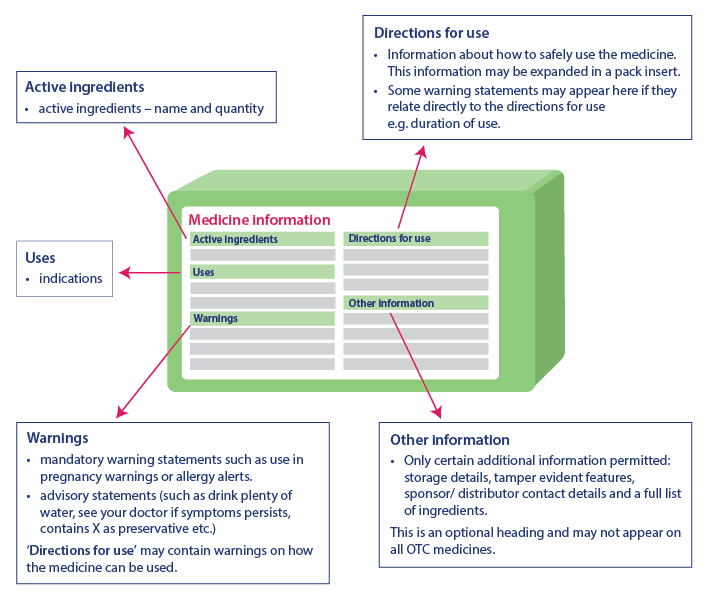
Critical health information helps consumers to use the medicine safely by providing important information. The information present in the critical health information table will always be in the same order, although there may be a slight difference in the representation of the information. Critical health information has the following headings and information in the same order:
- Active Ingredients: Name of the active ingredient and its quantity
- Indications: Usage of medicine
- Warnings: Includes warning statements, advisory statements and additional warning about the usage of the medicine under ‘Direction For Use’
- Directions For Use: Includes information about the safe usage of the medicine
- Other Information: Includes information such as storage details, tamper-evident features, sponsor contact details, list of ingredients, etc. This part may not appear on every medicine
Source: TGA
4. Pharmacy Dispensing
According to the new rule, the following changes are made to the medicine dispensers:
- To improve the identification of the medicines, the cartons of prescription medicines must display its name on the three non-opposing sides
- The labels of prescription medicines must have a dedicated space of 70x30 millimeters unless it is supplied in a primary or a starter pack
5. Allergen Information
Medicines must declare the following information on the labels:
- If present in the medicine, all the substances must be declared on the label
- Additional substances, such as crustacea, fish, eggs, soya, milk and tree nuts, must be declared on the label
- In the case of new allergens, manufacturers may not include them till the end of the 4 year transition period
Though the TGA has published this information for health professionals, we consider this as a critical requirement for manufacturers, too, to label their products comprehensively. Thus, health professionals can look at the updated information without any confusion and can guide the patients for safe consumption. It is well known that, the TGA has provided the manufacturers a transition period of four years to implement these changes. Post the defined date, the medicine labels must comply with the new labeling requirements. Stay informed. Stay compliant.









