
Compliance costs for pharmaceutical companies can account for up to 25% of their total operational expenses. Pharmaceutical companies with multiple product portfolios face unique challenges in managing global compliance, tracking dossier submissions, and overseeing labeling variations. The complexity of regulatory requirements across different regions, coupled with the manual handling of vast amounts of data, can lead to non-compliance, delays in market approvals, and operational inefficiencies.
This blog explores the key challenges pharmaceutical companies encounter while managing multiple product portfolios and demonstrates how regulatory software, backed by real-life case studies, offers a strategic solution to these problems.
Key Challenges in Managing Multiple Product Portfolios
- Complex Global Compliance Requirements: Each country has its own regulatory body, and their requirements can vary drastically. For example, the US FDA, EMA, and Japan’s PMDA have different submission formats, timelines, and compliance standards. Pharma companies must manage diverse dossiers across multiple regions while ensuring no deviation from local regulations.
- Tracking Label Variations Across Regions: Managing label variations for different regions and ensuring timely updates is one of the most challenging aspects of portfolio management. Mismanaging label changes can lead to non-compliance, product recalls, and major financial setbacks.
- Dossier Submission Overload: Submitting and managing dossiers across multiple product lines, each at different stages of development, can quickly overwhelm internal teams. Handling product lifecycle updates manually often leads to mistakes and delayed approvals.
- Resource Mismanagement: Teams often struggle to allocate the right resources efficiently. Without a robust management system, companies may overburden staff with administrative tasks, limiting their capacity to focus on core strategic activities.
- Data Silos: Lack of centralization means data related to submissions, approvals, and product updates are stored in disparate systems, leading to inefficiencies, miscommunication, and difficulty in maintaining a holistic view of the product lifecycle.
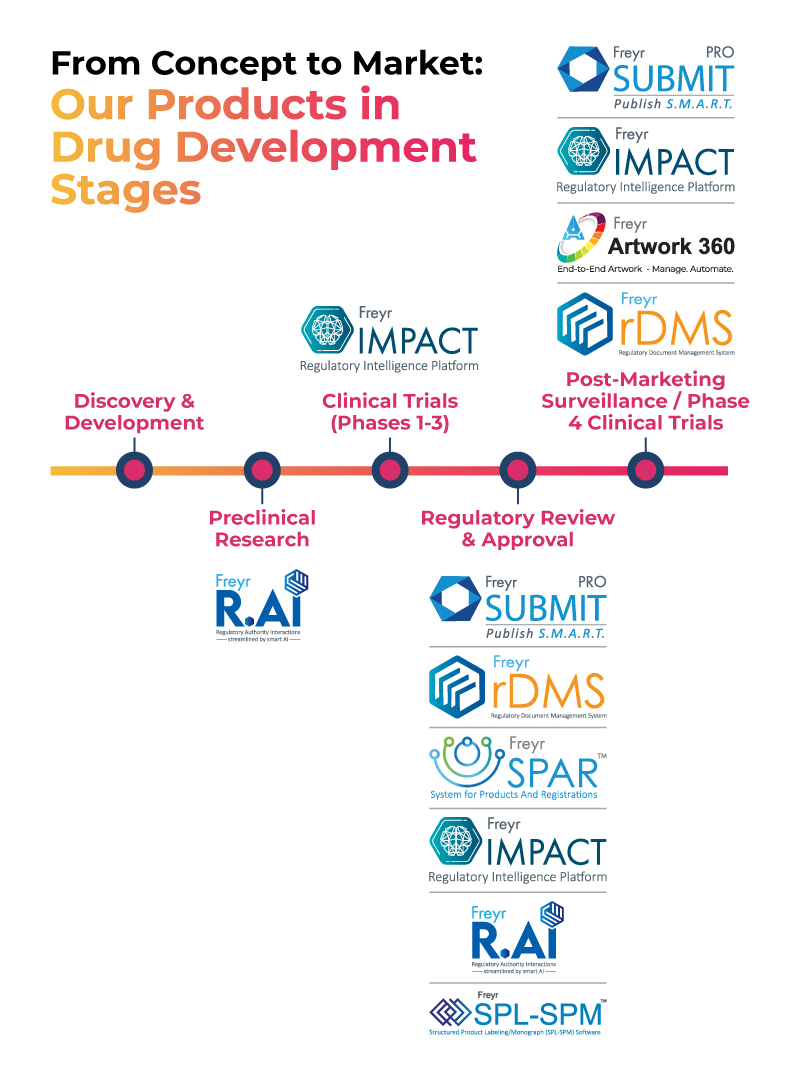
Freyr Digital's technological product suite supports life pharma companies from concept to market, as shown in the image below.
Case Studies: How Regulatory Softwares Provides Strategic Advantage
By embracing regulatory software, pharmaceutical companies can automate processes, ensure compliance, and ultimately gain a strategic advantage over competitors. The following case studies highlight real-world examples of how our regulatory software solutions have helped pharmaceutical companies navigate these challenges.
Proven Case
Client Challenge
A US-based generic pharmaceutical company was struggling to efficiently manage its end-to-end regulatory submissions and document management. They faced challenges with scattered document repositories, making it difficult to provide seamless access to regulatory documents for both internal and external stakeholders. Additionally, the process of creating and managing Structured Product Labeling (SPL) was cumbersome, leading to delays in submission timelines and increased review cycles.
Freyr Digital’s Solution
Freyr Digital stepped in as the virtual regulatory affairs solution's provider, deploying these three key products as solutions to address the client’s challenges:
- Freyr rDMS: A centralized document management system to act as the single source of truth for all regulatory submissions and documents, ensuring real-time access and version control for both internal teams and external partners.
- Freyr SUBMIT PRO: A powerful tool for eCTD publishing and submission, streamlining the submission process, reducing preparation time, and ensuring compliance with evolving regulatory standards.
- Freyr SPL-SPM: An efficient solution for managing all SPL-related activities, significantly improving document creation, management, and submission processes.
Results
By implementing these solutions, Freyr Digital helped the client achieve:
- A 30% reduction in submission preparation time
- 100% compliance with all regulatory requirements
- A 40% improvement in document processing efficiency
- A 25% reduction in review cycles
Conclusion: Embracing Digital Transformation for Strategic Success
Managing multiple product portfolios across global markets is no small feat. However, with the right regulatory software, pharmaceutical companies can streamline processes, ensure compliance, and gain a strategic advantage in the industry.
Freyr Digital’s suite of regulatory tools-including Freyr SUBMIT PRO, Freyr rDMS, and Freyr SPL-SPM -has consistently helped pharmaceutical companies reduce submission times, improve compliance, and optimize resources. By automating manual processes, providing centralized data management, and offering real-time insights, our solutions empower pharma companies to stay ahead in a competitive market. Contact us.









