Patient recruitment is a critical component of clinical trials, influencing both the timeline and the success of the clinical study. Inadequate recruitment can lead to delays, increased costs, and potential failure to gather sufficient data for Regulatory approval. Despite its importance, patient recruitment remains one of the most challenging aspects of clinical trial management. The blog explores effective strategies to enhance the patient recruitment process, addressing common challenges and highlighting the role of Regulatory partners.
Recruitment Challenges:
Recruiting patients for clinical trials is burdened with difficulties. Various factors contribute to these challenges including:
- Lack of Awareness: Many potential participants are unaware of clinical trials and their benefits.
- Patient Distrust: Historical misuses/ exploitations in medical research have led to a general distrust among some patient populations.
- Stringent Eligibility Criteria: Overly strict inclusion and exclusion criteria can limit the pool of eligible participants.
- Logistical Barriers: Geographical constraints and the burden of frequent site visits can deter participation.
Effective Patient Recruitment Strategies:
To overcome these challenges, several innovative strategies can be employed:
Patient-Centric Approaches
Involving patients in the trial design process can make studies more appealing and accessible. Understanding patient needs and preferences can reduce dropout rates and increase participation.
E.g., simplifying consent forms and providing clear, concise information can make the process less intimidating.
Digital Recruitment
Utilizing social media, online platforms, and patient databases can help reach a broader audience. Digital tools can target specific or special populations, making the recruitment process more efficient.
Community Engagement
Partnering with community organizations and healthcare providers can build trust and awareness. Local events, educational seminars, and collaborations with patient advocacy groups can boost clinical recruitment by making clinical trials more visible and trusted within communities.
Simplifying the Process
Streamlining the recruitment process by reducing the complexity of consent forms and providing clear, concise information can help. Offering flexible scheduling can also mitigate logistical challenges, making it easier for patients to contribute in clinical trials.
Use of Technology
Implementing electronic health records (EHR) and artificial intelligence (AI) can identify potential participants quickly and accurately. These technologies can analyze large datasets to find patients who meet the trial's eligibility criteria, speeding up the recruitment process.
Table 1: Traditional vs. Innovative Recruitment Approaches
| Traditional Recruitment | Innovative Recruitment |
|---|---|
| In-person advertising | Social media ads |
| Healthcare provider referrals | Online patient databases |
| Community outreach | Telehealth consultations |
Facilitating effective recruitment: Role of Regulatory Experts
Regulatory partners play a pivotal role in implementing innovative recruitment strategies. They provide expertise in:
- Digital Transformation: Guiding the integration of new technologies into recruitment processes.
- Regulatory Intelligence: Offering up-to-date insights on global Regulatory trends.
- Strategic Planning: Helping pharma align recruitment strategies with long-term business goals.
Regulatory experts ensure that recruitment practices adhere to Regulatory requirements while maximizing efficiency and effectiveness.
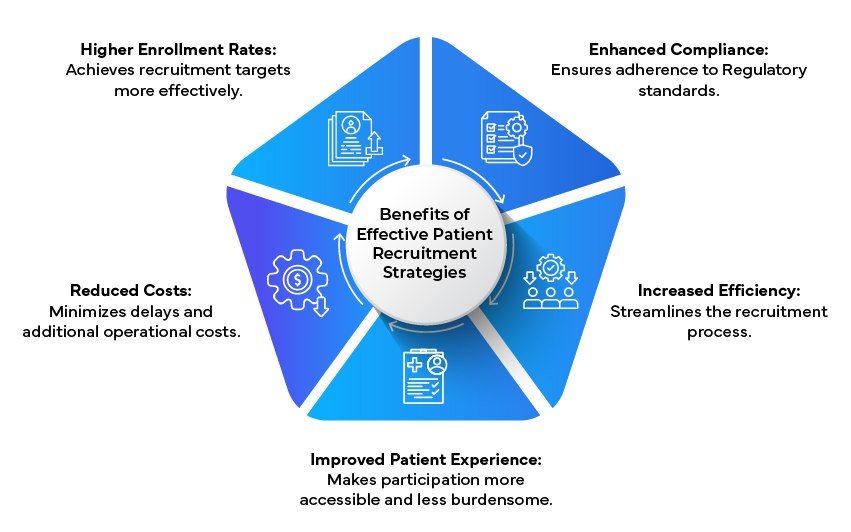
Conclusion
Effective patient recruitment is vital for the success of clinical trials. By adopting patient-centric approaches, leveraging technology, and collaborating with Regulatory experts, sponsors can overcome recruitment challenges and enhance trial outcomes. These strategies not only improve the efficiency and effectiveness of patient recruitment but also ensure that clinical trials are conducted in a manner that respects and prioritizes patient needs.