
As the use of pharmaceuticals can have a substantial impact on a patient's health and well-being, the information on the label must be clear and correct. It is particularly true when it comes to usage directions, expiration dates and component lists. Thus, an audit of pharmaceutical labeling operations is required.
As a significant aspect of Regulatory compliance, data accuracy stands as one of the most critical criteria in medicine labeling. According to the FDA and MHRA studies, roughly 51 percent of auditing issues are associated with labeling-related documents.
The five (05) most common audit issues that were highlighted by the major Health Authority label audit findings are as follows:
- Deviation from SOPs
- CCDS & CSI Information
- Version Control
- Ineffective Tracking
- Decentralization
The above-listed issues may arise at the time of mergers and acquisitions, multiple products approved in multiple countries, and changes in local labeling.
Top Reasons for Pharma Labeling Audit Issues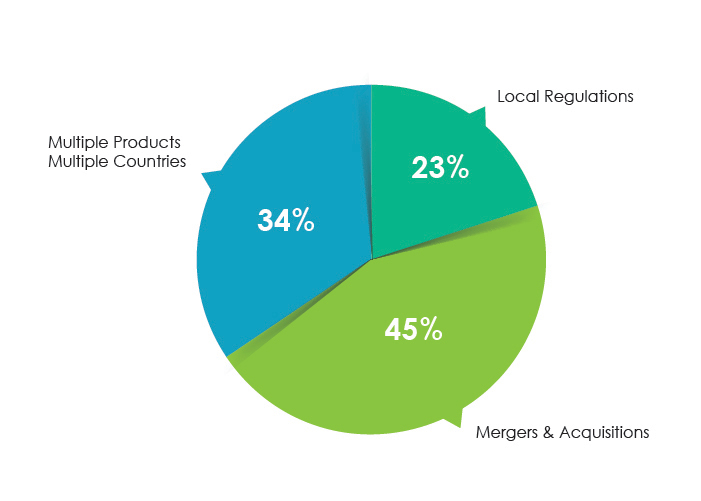
Failure to fulfill the requirements of Regulatory standards to disclose product safety information, or sharing such information can:
- Put the patient safety at risk: Any inaccuracies in labeling could result in the misuse of a drug, resulting in negative effects and, in some cases, may be fatal.
- Be costly to organizations: Recalling a product due to a labeling error is a costly process that can harm a company's bottom line either the profitability or brand image.
- Be Time Consuming: When there is a labeling information gap, investigating a labeling issue may be time consuming for Health Authority
- Damage the Reputation: Once the product is recalled from the market, it may even impact the brand image and subsequently can have a detrimental impact on an organization’s reputation.
In such scenarios, how do you ensure that your pharmaceutical product labeling procedure will meet the required criteria?
Adopting Automation for Labeling Compliance
Automation, like it does in so many other fields, is critical in ensuring global labeling compliance. In addition to this, automation reduces human intervention and ensures the development of high-quality deliverables that significantly reduce overall operational costs and improve the time-to-market of organizational product lines.
Today, the best method to successfully meet Regulatory labeling compliance is to invest in a comprehensive label life cycle management tool. By contracting out global labeling operations to specialized end-to- end label management software vendors, pharmaceutical manufacturers can bank on effective and streamlined label management.









