Under the Classification, Labeling and Packaging of substances and mixtures (CLP) regulation, Chemical companies, who wish to place hazardous chemical mixtures such as paints, coatings, detergents, solvents, etc. in the European market are required to provide notifications of the hazards in their products. Poison centres take the responsibility to collect relevant information about hazardous mixtures and provide medical advice during health emergencies.
With various notification systems and information requirements across different countries in the EU, ANNEX VIII of the CLP Regulation was implemented. It aims to harmonize the hazardous information and the format that must be submitted to poison Centres to improve emergency responses.
Notification Deadlines
Article 45 of the CLP Regulation describes the notification obligations for importers and downstream users who want to place hazardous chemical mixtures in the EU market.
- The first deadline ended on 1st January 2021 for mixtures classified as hazardous for consumer use and professional use products. Therefore, companies manufacturing these products must comply with the new harmonized PCN format.
- Mixtures intended for industrial use must comply from 1st January 2024.
- Mixtures that are already placed in the market and notified under the national legislation must comply from 1st January 2025.
The earliest date applies if a mixture has more than one of the above uses either for direct use or for manufacturing further down the supply chain. The mixtures are subject to existing national information requirements before the relevant date of applicability.
If there is a change to the mixtures that are already notified under the national legislation, companies must send notifications based on the latest information requirements according to Annex VIII.
New Harmonized Format
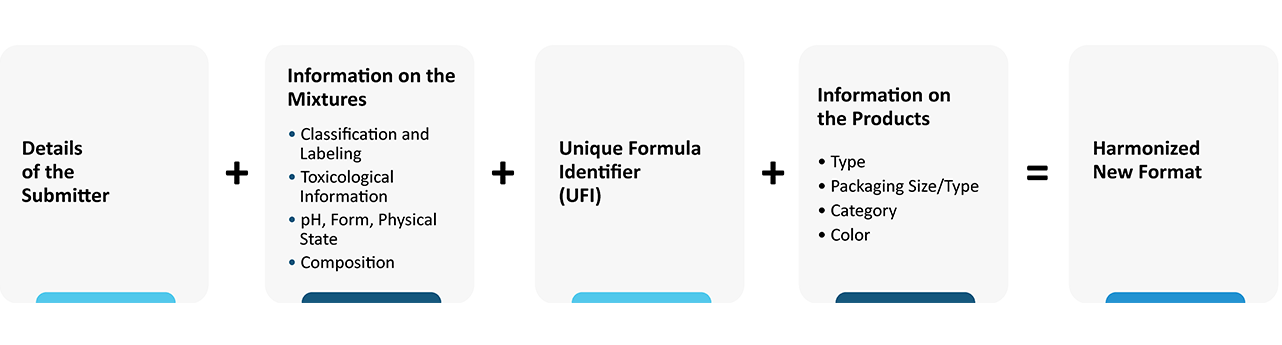
Companies, who wish to submit the Poison Centre notification, must provide the required information using the new harmonized format. The format is XML-based and compatible with IUCLID. The new harmonized format must contain the following information:
- Details of the submitter
- Detailed information on the mixtures such as Classification, Labeling, Toxicological Information, pH, Form, Physical State, Composition, etc.
- Unique Formulation Identifier (UFI)
- Product information such as type, packaging size, category, color, etc.
Failing to submit the required information in the new format within the specified deadlines will result in applicable fines or banning the product from the market. Hence, it is crucial to analyze your entire product portfolio and optimize your data management system to the harmonized PCN format before the relevant deadlines. To avoid hassles and achieve compliance, reach out to a Chemicals Regulatory Submissions expert.