
The Electronic Common Technical Document (eCTD) has become the golden standard for drug regulatory submissions worldwide, and with the advent of eCTD 4.0 in 2022, a new chapter of innovation began. While this latest version boasts remarkable benefits like heightened efficiency and precision, it also ushers in unique challenges for countries transitioning to this cutting-edge format. In this blog, we'll embark on a journey to uncover these challenges, as the world navigates the transformative waters of eCTD 4.0.
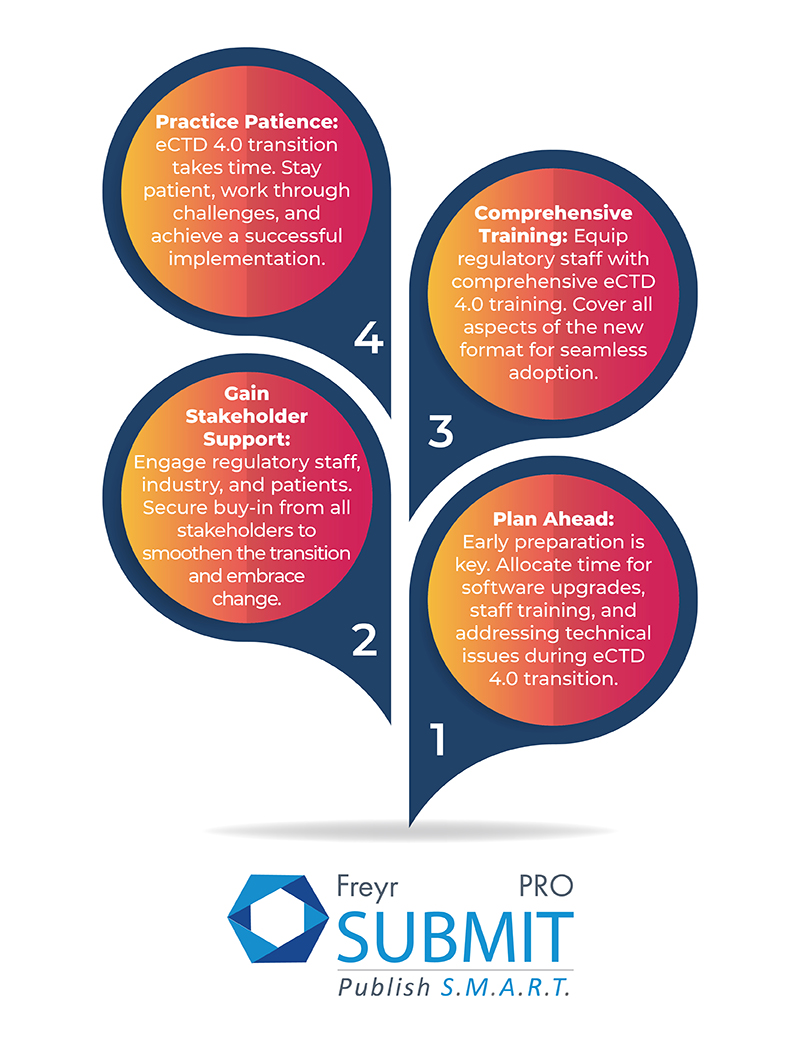
- One of the biggest challenges is the need for a new template. eCTD 4.0 submissions cannot be compiled with the previous versions of eCTD templates, so countries that want to submit eCTD 4.0 submissions will need to upgrade their software. This can be a costly and time-consuming process.
- Need for training could be another challenge. The new features and improvements in eCTD 4.0 can be complex, so countries will need to train their regulatory staff on how to use the new format. This can also be a costly and time-consuming process.
- Countries will need to ensure that their infrastructure is compatible with eCTD 4.0. This includes having the necessary hardware and software in place, as well as having a reliable internet connection.
- There is a lack of awareness of eCTD 4.0 among Regulatory staff and stakeholders, it can be difficult to get everyone on board with the transition.
- There might be technical issues with the new format that need to be resolved before countries can start submitting eCTD 4.0 submissions.
Transitioning to eCTD 4.0 may present its share of challenges, but they are conquerable with dedication and determination from countries. While significant investment and effort will be required, the rewards that eCTD 4.0 brings are equally substantial, making the endeavor truly worthwhile.
Amidst these challenges, countries can take proactive steps to pave a smoother path toward eCTD 4.0 adoption. By following purposeful efforts and strategic approaches, they can unlock the full potential of this innovative standard and usher in a new era of Regulatory excellence.
Transitioning to eCTD 4.0 might seem like a daunting task, but fear not! With the right strategy and guidance, countries can conquer this challenge and unlock the full potential of eCTD 4.0's wonders. Partnering with vendors like Freyr, who have a reach in about 20 countries around the world and are well-versed in Regulatory publishing, is the key to success for life sciences organizations. At Freyr, we take pride in driving innovation through technology, and our Regulatory submission and publishing software, Freyr SUBMIT PRO. Got quick queries on eCTD submissions? Contact Us today and pave the way for a seamless Regulatory future!









