
DYK? Australian Regulatory framework for therapeutic goods has been evolving with regards to labeling requirements. It is clearly evident with the Therapeutic Good Administration’s (TGA’s) recent announcements and proposed labeling requirements. Recently, it has announced certain guidelines for using the ‘TGA assessed claim’ on medicine labels. Aimed at the sponsors, these guidelines are targeted towards both assessed listed and registered complementary medicines. These guidelines clearly detail the usage of the TGA symbol and the TGA-compliant statement on a medicinal label. What exactly these mean to the sponsors? Let us deep dive.
What Does the “TGA Assessed Claim” Mean?
The TGA assessed claim is a symbol and/or statement that describes that medicine has efficacy for the indications claimed and is assessed by the TGA. The TGA assessed claim must be displayed as the TGA assessed statement with or without the ‘TGA assessed’ symbol. But if the symbol is used, it should be displayed with the statement. The wordings in which the statement is presented is also important for compliance. The TGA requires these to appear in the below-mentioned way.
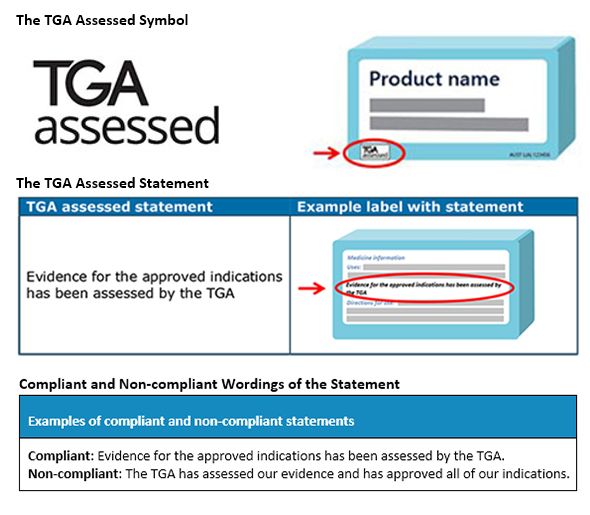
As these assessed claims are required to be presented on ‘listed assessed medicines and registered complementary medicines,’ the Sponsors should clearly evaluate what do they require prior to using their symbol and statements. The Sponsors must apply for using the TGA Assessed Claim for their medicines. If in the claim, the presentation of the medicine is not acceptable as per legislative requirements, the authorization won’t be provided. At times, to understand the complete perspective of TGA assessed claims, consulting a regional Regulatory expert is all that is required. Stay informed. Stay compliant.









