Toxicology studies are foundational to the drug product approval process, ensuring that new pharmaceuticals are safe for human use. These studies assess the potential adverse effects of new compounds and determine safe dosage levels. Without thorough toxicology evaluations, the risks associated with new drug products could outweigh their benefits, leading to severe consequences for patients and setbacks for pharmaceutical companies.
Despite their critical importance, toxicology studies can be associated with challenges. Inadequate or flawed toxicology assessments can lead to significant setbacks in drug development, including clinical hold-ups, additional testing requirements, or outright denial of approval by Regulatory authorities. These issues not only delay the availability of potentially life-saving medications but also increase development costs and reduce the likelihood of a drug reaching the market.
Key Aspects of Toxicology Studies
Toxicology studies encompass various types, each addressing specific safety concerns relevant to pharmaceuticals. Here, we explore the primary types of toxicology studies and their roles in the drug product approval process.
Preclinical Toxicology
Preclinical toxicology involves initial studies conducted in vitro and in vivo to evaluate toxicity levels before human/ clinical trials. These studies help identify potential adverse effects and determine safe dosage ranges.
Chronic Toxicity Studies
Chronic toxicity studies assess the effects of prolonged exposure to a drug, which is crucial for treatments intended for chronic diseases. These long-term studies help identify any delayed adverse effects that may not be apparent in short-term studies.
Reproductive and Developmental Toxicology
These studies examine the potential effects of a drug on reproduction and fetal development. They are essential for ensuring that drug products do not adversely affect fertility or cause developmental issues in offspring.
Genotoxicity Testing
Genotoxicity tests determine if a compound can cause genetic mutations, which could lead to cancer. These assessments are vital for identifying potential carcinogenic risks associated with new drugs.
| Toxicology Study Type | Purpose |
|---|---|
| Acute Toxicity | Determines immediate effects of a drug |
| Sub-chronic Toxicity | Assesses effects of repeated exposure over a short period |
| Chronic Toxicity | Evaluate the long-term effects of prolonged exposure |
| Reproductive Toxicology | Examines drug impact on fertility and fetal development |
The Role of Regulatory Experts
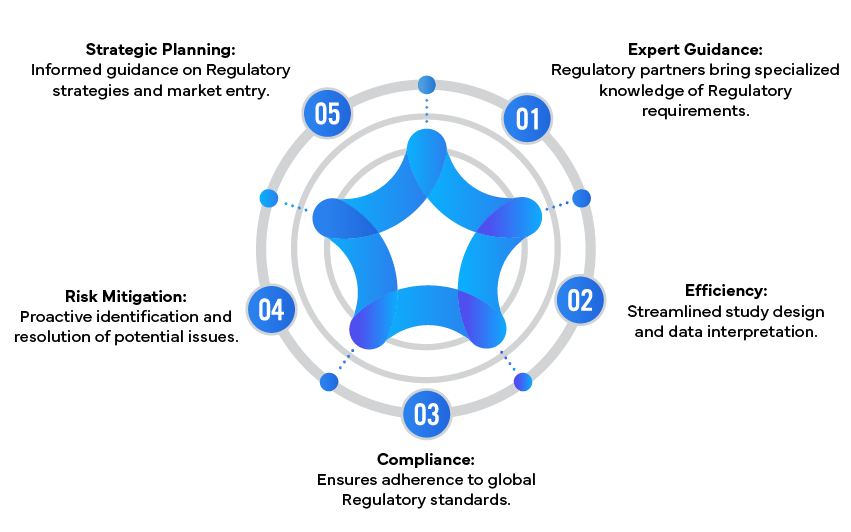
Regulatory experts play a pivotal role in ensuring the success of toxicology studies. They provide expertise in designing and interpreting these studies, ensuring compliance with global Regulatory standards. Regulatory partners assist in preparing comprehensive reports for submission and addressing Regulatory queries effectively. Their involvement can streamline the approval process and enhance the likelihood of a drug's success.
Benefits of Collaborating with Regulatory Partners
Conclusion
Toxicology studies are critical in establishing the safety profile of new drug products. By addressing various safety concerns through different types of toxicology assessments, these studies ensure that new pharmaceuticals are safe for human use. Collaborating with experienced Regulatory experts can further streamline the approval process, ensuring adherence to stringent safety standards and enhancing the likelihood of a drug product’s success in the market. By adopting a comprehensive and proactive approach to toxicology, pharmaceutical companies can navigate the complexities of drug product development more effectively, ultimately bringing safer and more effective treatments to patients.