With an increasing number of new drug applications and clinical studies conducted to substantiate the efficacy and safety of new molecules, it becomes critical for pharmaceutical organizations to disseminate scientific information to the target audience via publication in a renowned scientific journal. Knowledge advancement, the ability to leverage existing knowledge in new ways, and its execution merely define the quality of the scientific writing directed to the target audience, including the Regulatory fraternity. A high level of understanding and vetting is required to write and review scientific documentation about a specific molecule.
The American Medical Writing Association (AMWA) and the European Medical Writing Association (EMWA) are the educational organizations that developed the code of ethics to be adhered to by scientific writers. To maintain standards of Good Publication Practice (GPP3), the International Society for Medical Publication Professionals (ISMPP) and the International Committee of Medical Journal Editors (ICMJE) have put forward guidelines and a structured format to be followed by pharmaceutical organizations.
Ethical Issues in Scientific writing
The Life Sciences industry understands scientific writing as a medium that deals with creating high-quality technical content for the scientific community. It addresses science-related issues that include novel discoveries of theory, mechanism, and synthesis of various chemicals in the biopharmaceutical industry. However, sometimes the published scientific information is unethically duplicated for financial benefit.
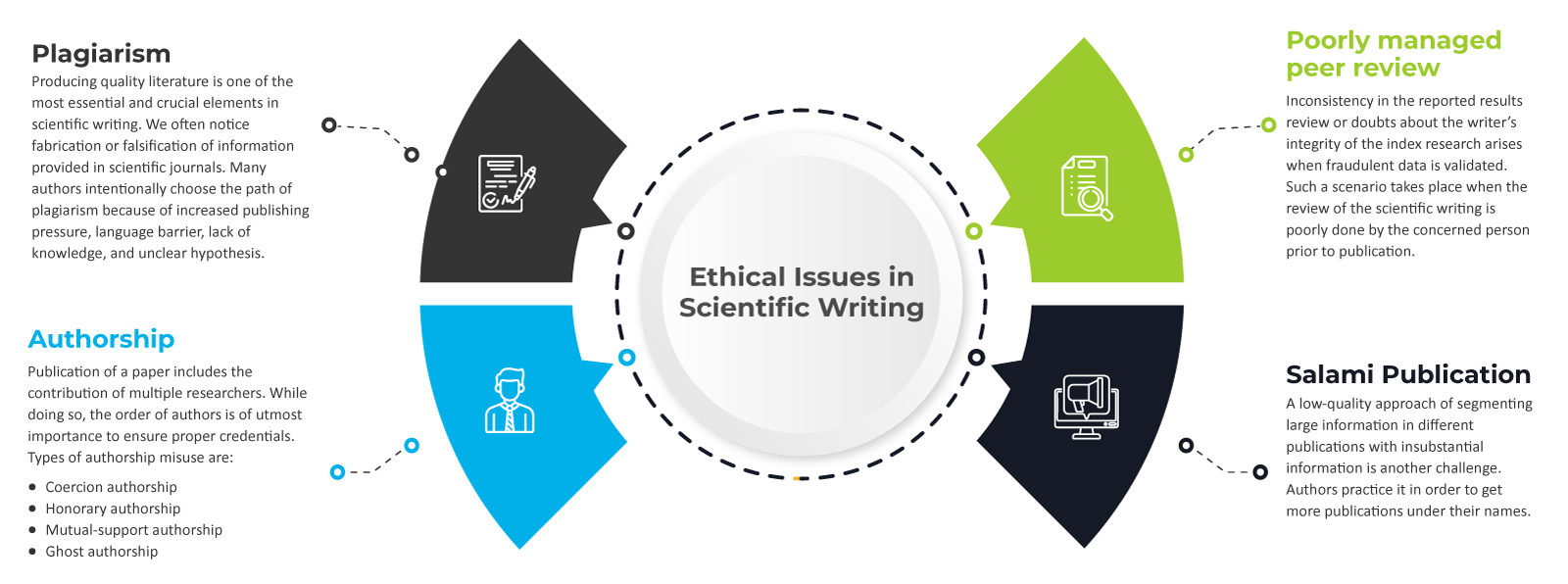
Persevere the Scientific Writing Integrity
“Science knows no country because knowledge belongs to humanity and is the torch which illuminates the world.” - Louis Pasteur
A scientific writer requires skills such as proper understanding, interpreting, summarizing complex scientific and statistical data via scientific journals, literature, etc., in providing effective guidance to other scientists as well as researchers. Following the age-old Introduction, Methods, Results, and Discussion (IMRAD) structure allows writers to organize their manuscripts effectively.
An excellent scientific writer is capable of:
- Having clarity about the purpose of the trial
- In-depth understanding of the research
- Potential to reflect and organize the ideation of the writing
- Ability to communicate the research findings in their writing
A scientific manuscript gets rejected when there is a lack of originality, relevance, and inadequacy in reported results. Insertion of voluminous content in manuscripts deviates the focus from the objective, making the entire piece difficult to interpret. A thorough review of the manuscript ensures proper formatting of the results, avoids grammatical or language errors, and improves the research communication quality. For compliance, reach out to a team that ensures the information curated is in conjunction with the requirements of the shortlisted journal. Contact Freyr today.