
Emergency Use Authorizations (EUAs) are significant in making life-saving medicinal products available to patients faster. They have been helpful in dealing with pandemics like COVID-19. An initiative of the United States Food and Drug Administration (USFDA) started in the year 2004, and the EUA came into force when Section 564 of the Federal Food, Drug, and Cosmetic Act was amended by the Project BioShield Act. This program is a step taken by the FDA to protect public health by ensuring the safety, efficacy, and quality of medical products while addressing medical emergencies and emerging threats to public health.
Let us understand it better in the following lines.
Decoding the EUA
The EUA pathway is a means to facilitate the accessibility of medical countermeasures in times of declared emergencies. The Commissioner of the FDA can authorize the below in such situations:
- The authorized use of unapproved medical products.
- The unauthorized use of approved medical products.
The products that are covered under the EUA include vaccines, IV fluids, drugs, devices, tests, etc., and can be used to diagnose, treat, or prevent life-threatening conditions. Products are granted EUA if the following criteria are met:
- Proof of a life-threatening condition/illness.
- The scientific data provides enough evidence that the product is effective for its intended use.
- The benefits of the product outweigh the risks (aka evidence of safety).
- Lack of alternative products.
Sponsors are advised to understand the FDA’s requirements in advance so that they follow the best Regulatory process and ensure an error-free EUA submission. Following is mandatory information that needs to be shared by the sponsor in the application.
Data to be Submitted by the Sponsor to the FDA for a EUA Approval
- Product description and its intended use.
- The approval status of the product with the FDA.
- Safety and efficacy information such as clinical and non-clinical data, etc.
- Risk-benefit analysis report.
- Chemistry, Manufacturing, and Controls (CMC) data.
- Information on the dosage, contraindications, warnings, and adverse events for the distribution of the medical product in question.
How are EUAs Issued by the FDA?
Below is a diagrammatic interpretation of the EUA lifecycle in brief:
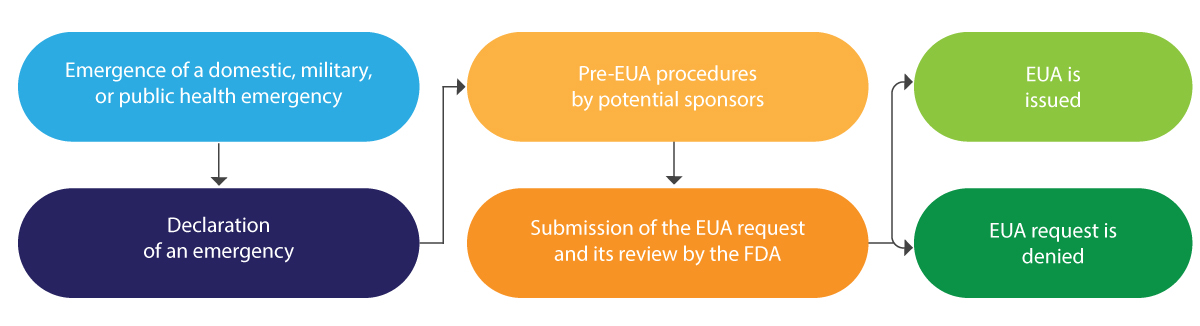
The EUA is typically issued for a limited period and at the end of the said emergency, it is terminated by the FDA.
EUA and COVID-19
The Secretary of Health and Human Sciences (HHS) declared COVID-19 as a pandemic on January 31, 2020. Since then, the FDA has been instrumental in approving a few vaccines and home test kits under the EUA pathway to deal with the ongoing global outbreak.
With the emergence of new variants of the COVID-19, the entire pharmaceutical industry is working hard to curtail the spread and lower the mortality rates. There is a need for novel medicinal products and faster approvals by global Regulatory Authorities so that their time-to-market is reduced. The EUA route of registering new medicinal products/drugs is the way forward, according to experts. Several other Health Authorities like the European Medicines Agency (EMA), the Central Drugs Standard Control Organization (CDSCO), the Saudi Food and Drug Authority (SFDA), etc., have also implemented the EUA pathway for faster approvals.
If you are a drug manufacturer and are looking for a EUA for your life-saving product, you will need an adept Regulatory solutions provider. Reach out to Freyr for a faster time-to-market and a compliant pathway.









