In the pharmaceutical industry, patient safety and Regulatory compliance are paramount. One critical aspect of ensuring both is the assessment and control of genotoxic impurities in drug products. The International Council for Harmonization (ICH) M7 guideline provides a comprehensive framework for managing these impurities, which can cause genetic mutations and lead to cancer. Genotoxic impurities, even in trace amounts, pose significant risks to patients. These impurities can cause genetic mutations, potentially leading to cancer. Therefore, proper identification, risk assessment, and control of genotoxic impurities are essential to mitigate these risks and ensure the safety and efficacy of pharmaceuticals. This blog explores the key aspects of ICH-M7, the challenges it addresses, and the role of Regulatory experts in achieving compliance.
Key Aspects of ICH-M7
The ICH-M7 guideline outlines a structured approach to the assessment and control of genotoxic impurities. It encompasses several key components:
Risk Assessment
Risk assessment involves identifying potential genotoxic impurities and evaluating their risk based on their chemical structure, data from similar compounds, and available toxicological data. This step is crucial for determining which impurities need to be controlled and at what levels.
Control Strategies
Once potential genotoxic impurities are identified, control strategies must be implemented to limit their presence within acceptable limits. This includes developing and validating analytical methods to detect impurities and implementing manufacturing controls to minimize their formation.
Regulatory Submission
Comprehensive documentation and justification of risk assessments and control strategies are required for Regulatory submissions. This ensures that Regulatory bodies have all the necessary information to evaluate the safety of the drug product.
Table 1: Components of ICH-M7
| ICH-M7 Component | Description |
|---|---|
| Hazard Assessment | Identifies and categorizes impurities based on their genotoxic potential |
| Risk Characterization | Assesses exposure levels and potential risks to patients |
| Control Measures | Strategies to limit or eliminate impurities during production |
(Not limited to)
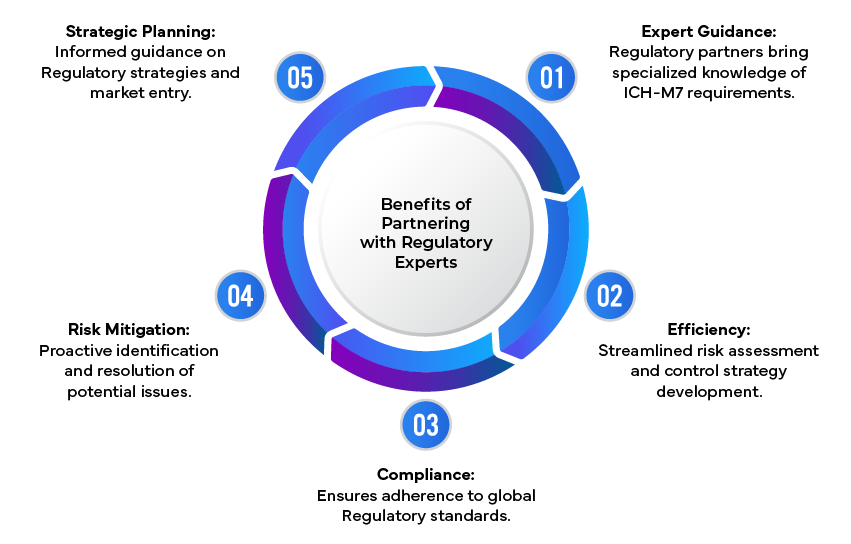
The Role of Regulatory Experts
Navigating the complex ICH-M7 requirements can be challenging for pharmaceutical companies. This is where Regulatory experts play a vital role by providing expert guidance on risk assessments, control strategies, and the preparation of comprehensive Regulatory submissions. By leveraging their expertise, companies can ensure compliance with ICH-M7 and mitigate potential risks associated with genotoxic impurities.
Summary
ICH-M7 guideline compliance is essential for ensuring the safety and efficacy of pharmaceuticals. By adopting a structured approach to the assessment and control of genotoxic impurities, companies can protect patient safety and meet Regulatory requirements. Partnering with Regulatory experts further streamlines this process, providing the necessary expertise to navigate complex Regulatory landscapes and achieve successful submissions.