
In the dynamic world of pharmaceuticals, the role of real-world data (RWD) and real-world evidence (RWE) is becoming more pivotal. These tools provide a bridge between the controlled environment of clinical trials and the real-world complexities of patient care. Several pharmaceutical companies have successfully used RWD/RWE to support their regulatory submissions. For example, in 2022, Bristol Myers Squibb received FDA approval for the use of Opdivo (nivolumab) in combination with Yervoy (ipilimumab) for the treatment of first-line metastatic melanoma based on RWE data from the CheckMate 151 trial.
With the recent FDA draft guidance in October 2023 paving the way, it's time for pharmaceutical innovators to harness the potential of RWD and RWE for regulatory submissions.
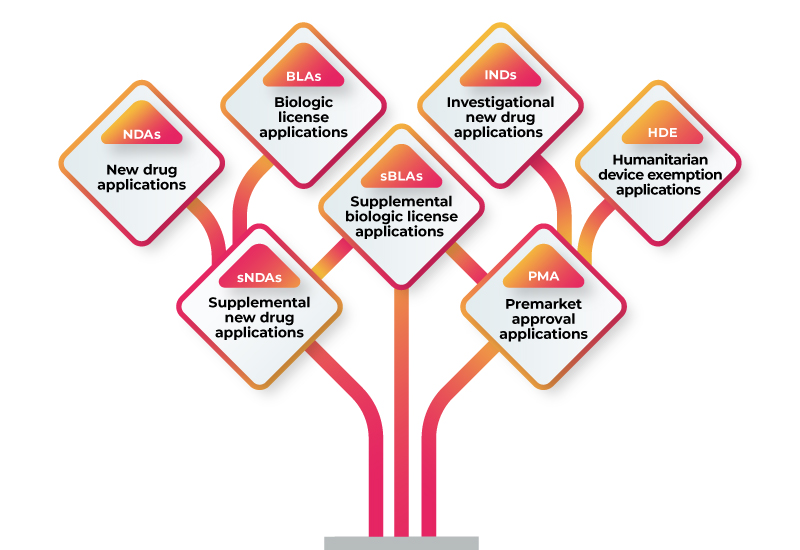
RWD/RWE can be used to support a variety of regulatory submissions, including:
To use RWD/RWE to support your regulatory submission, you should follow these steps:
Step 1: Define Your Regulatory Question
Begin your journey by identifying the core regulatory question you aim to address. Whether it's safety, efficacy, or performance in real-world scenarios, clarity in your goal is paramount.
Step 2: Select Quality RWD Sources
Quality is the key here. Choose RWD sources that directly correlate with your regulatory question. Electronic health records, medical claims data, and patient registries are invaluable resources.
Step 3: Craft a Sound Study Design
Your study design should mirror your regulatory question and adhere to regulatory guidelines. Your approach must align with the FDA's expectations.
Step 4: Analyze and Generate RWE
Use robust statistical methods for your analysis. Clear documentation is essential to ensure transparency and credibility.
Step 5: Interpret and Present Your Findings
Objectivity is the cornerstone of RWE interpretation. Your presentation should be tailored to your audience, conveying the totality of evidence with precision.
Here are some tips for using RWD/RWE to support your regulatory submission:
- Complement, Don't Replace Clinical Trials: RWD/RWE enhances safety and efficacy evidence but doesn't replace well-designed clinical trials.
- Transparent Limitations: Acknowledge RWD/RWE's observational nature, potential biases, and uncontrolled confounding factors.
- Collaborate with Regulatory Agencies: Work closely with regulatory agencies to align your RWD/RWE use with their expectations.
By following these steps and tips, you can use RWD/RWE to support your regulatory submission and bring your product to market more quickly and efficiently.
As you embark on this RWD and RWE journey, remember that you're paving the way for more efficient and informed regulatory submissions. The future of pharmaceutical innovation lies in real-world evidence. Embrace it, harness it, and make your mark in the industry.
Ready to streamline your regulatory submission process? Explore our cutting-edge regulatory submission software Freyr SUBMIT PRO today!









