Medical Device Registration in UAE Overview
United Arab Emirates (UAE), a prominent GCC member Nation, has an advanced healthcare system. Its market potential is proven and consistently on a rise, governed by the Drug Control Department of the Ministry of Health and Prevention (MOHAP). Centralized governance and linguistic barriers are major hinderances for medical device registration in UAE, along with linguistic complexities and lack of efficient communication channels with the Health Authorities.
Regulatory Authority: Drug Control Department of the Ministry of Health and Prevention (MOHAP)
Regulation: UAE Medical Devices Registration Guideline
Regulatory Pathway: Product Registration
Authorized Representative: UAE Local Authorized Representative is required
QMS Requirement: ISO 13485:2016
Assessment of Technical Data: Medical Device Registration Committee
Validity of License: 5 Years
Labeling Requirements: Annex 2 (2.5) of UAE Medical Devices Registration Guideline
Submission Format: Paper
Language: English
UAE Medical Device Classification
UAE has separate Classification Rules for Medical Devices and IVDs. The UAE medical device classification rules are in line with the classification rules of the EU Medical Device Directives. The class of devices as per the UAE classification rules are as below
| Risk Criteria | Medical Device Class |
|---|---|
| Low Risk | I |
| Low Moderate Risk | IIa |
| Moderate – High Risk | IIb |
| High Risk | III |
| Risk Criteria | IVD Class |
|---|---|
| Low Individual Risk and Low Public Health Risk | A |
Moderate Individual Risk and/or Low Public Health Risk | B |
High Individual Risk and/or Moderate Public Health Risk | C |
| High Individual Risk and High Public Health Risk | D |
UAE Local Authorized Representative
Foreign Manufacturers, with no physical office, shall appoint a Local Representative (LR) to act on behalf of them. The local representative should be licensed by the Ministry of Health as a medical store or scientific office (in case of scientific office, importation and distribution activities should be performed by an appointed Licensed Medical Store). The applicants may appoint their distributor as their Local Representative. However, having an independent Local Representative, with no commercial interest, would provide the required flexibility to appoint multiple distributors in the UAE. The details of both the LR and the distributor must be provided during device registration.
Official classification Process with UAE MoHAP
The UAE MoHAP has introduced an official classification service, particularly useful when you are unsure if your product requires registration. This service classifies products of all types and forms based on their presentation, composition, use, and design. Requirements may vary depending on the product's nature, risk class, and regulatory status.
The classification letter indicates whether a product needs to be registered with MOHAP. If registration is required, the product must be registered according to the class identified in the classification letter. This letter is valid for three years from the date of issue.
The official classification results can be:
- Does not require MOHAP registration
- Cleared by UAE MOHAP as a medical device, restricted to professional use
- Cleared by UAE MOHAP as an over-the-counter medical device
UAE Medical Device Registration
Certain Devices which do not require product registration or prior listing or approval for importation. Such products exempted from registration or listing shall apply and obtain an Import permit to be marketed in UAE.
For other devices, imports will not be cleared unless a pre-approval for importation of the consignment is issued by the DRCD. Such devices shall either be listed or registered to import into the UAE.
Listing of Devices: Generally, products used in hospitals under the professional supervision and Class I devices do not undergo detailed evaluation and they are required to be listed. A Listing Certificate will be issued by the agency. The devices after listing shall obtain import permit to market devices in the UAE.
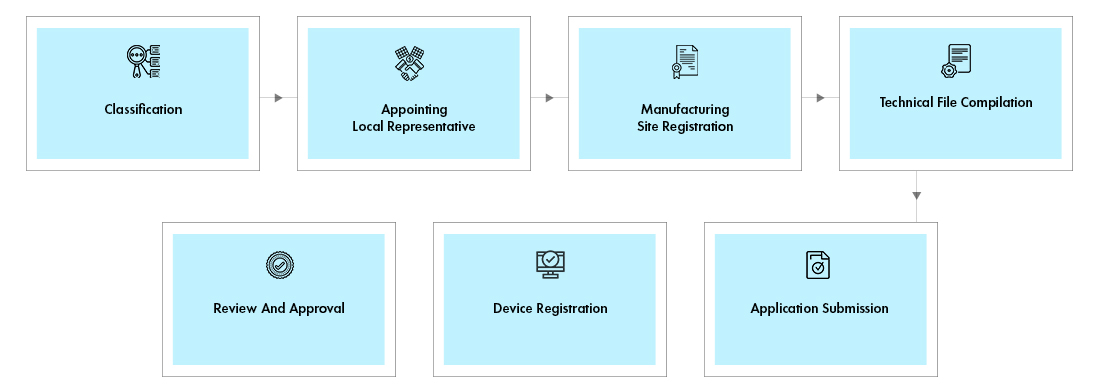
Registration of Devices: The Registration activity includes Site and Product Registration.
- Site Registration:The manufacturing site must be registered, if the device manufactured in that Site is being imported to the UAE for the first time. For the subsequent devices manufactured in the same site, only device registration would suffice, and site registration is not required.
- Device Registration:These devices are subject to review by the technical committee which upon approval will be granted a license certificate.
Process flow
Post Approval Device Life Cycle Management
- Post approval change management - modifications to existing Medical Device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between the MoH and the manufacturer
- Importation Management
With an exclusive delivery center in Dubai, Freyr holds an authoritative hold on the UAE Medical Device market and outlines the device classification apart from decoding the guidance regulations for better compliance. We support clients in document compilation as per the standards and thus ensure quick approvals. Freyr offers a complete range of Regulatory services pertaining to successful device marketing.
Summary
| Type of Device | Device Listing | Device Registration | Import License |
|---|---|---|---|
Device Exempted from Pre-Importation Approval (As listed in Annex 3) | NA | NA | YES |
| YES | NA | YES |
| All Other Devices | NA | YES | YES |
Freyr Expertise
- Regulatory Intelligence
- Regulatory Due Diligence
- Formal Medical Device Classification
- Device Registration
- UAE Authorized Representation
- Translation support
- Labeling support
- Distributor identification and qualification
- Post Approval Change Management
- License renewal and transfer
- Customs clearance