Frequently Asked Questions
Regulatory Affairs (RA) in the pharmaceutical industry involves ensuring the quality, safety, and efficacy of medicines throughout their lifecycle. RA professionals navigate complex global health authority requirements, maintaining compliance from drug discovery to commercialization. They act as a communication link between pharmaceutical companies and Regulatory authorities worldwide.
The primary purpose of regulation in the pharmaceutical industry is to safeguard public health by ensuring that new drugs are safe and effective before reaching the market. Regulatory agencies enforce rigorous standards for testing and evaluation, minimizing potential health risks. This Regulatory oversight provides confidence in the medical treatments available to the public.
Regulatory Affairs professionals ensure that pharmaceutical products comply with Regulatory standards throughout their lifecycle. They are involved in clinical trial design, marketing authorization, and post-approval management, ensuring ongoing compliance and facilitating necessary product changes. Their expertise helps companies bring safe and effective products to market efficiently.
Lifecycle management in pharmaceuticals involves maintaining the viability of a product from development through post-approval. It includes strategies for clinical development, marketing authorization, and ongoing compliance with Regulatory standards. Effective lifecycle management ensures the product remains safe and effective throughout its market presence.
Pharmaceutical regulation has evolved significantly, from medieval drug lists to modern international harmonization efforts. Key milestones include the US Pure Food and Drugs Act of 1906 and the formation of the International Conference on Harmonization (ICH) in 1990. These developments have strengthened global standards for drug safety and efficacy.
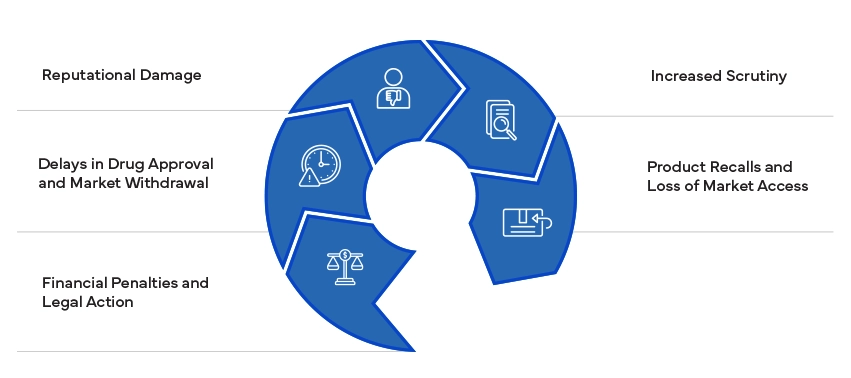
Non-compliance with Regulatory standards can lead to significant consequences, including fines, litigation, and product withdrawals. It can damage a company's reputation and result in increased scrutiny from Regulatory authorities. Ensuring compliance is essential to avoid these risks and maintain market access.
Freyr offers comprehensive Regulatory solutions, supporting clients from early-stage development through commercialization. Their services include preparing documentation for health authorities, identifying Regulatory challenges, and advising on post-marketing strategies. Freyr's expertise helps expedite product development and market availability.
Global Regulatory harmonization aims to streamline drug development and registration processes across different countries. It minimizes animal testing and ensures public health by aligning Regulatory requirements internationally. This harmonization facilitates the efficient introduction of new treatments to global markets.
Regulatory agencies such as the FDA in the United States and the EMA in the European Union enforce standards to ensure drug safety and efficacy. They oversee comprehensive testing and evaluation processes before drugs can be marketed. These agencies balance the need for new treatments with minimizing potential health risks.
Freyr's Global Regulatory intelligence framework provides up-to-date information on global regulations and Health Authority standings. This framework is integrated into customer/ client projects to enhance market-specific success. It supports strategic planning and Regulatory compliance across multiple geographies.