Innovative medical devices are essential for improving healthcare outcomes, addressing evolving medical needs, and providing cost-effective solutions for patients and healthcare providers. The SAKIGAKE strategy is a fast-track review and approval process for innovative medical devices in Japan. This strategy was initiated by Japan’s Ministry of Health, Labour and Welfare (MHLW) in 2015, with the aim of accelerating the development and commercialization of innovative medical devices in Japan.
Under the SAKIGAKE strategy, medical devices that meet certain criteria for innovation can receive priority review and approval by the Pharmaceuticals and Medical Devices Agency (PMDA), the Regulatory Agency responsible for medical device approvals in Japan. The designation criteria for innovation include the following:
- Devices with prominent effectiveness; the device is expected to provide significant clinical benefit with a novel mechanism of action and radical improvement to patients compared to existing medical devices.
- Firstly, the device is applicable for approvals in Japan or simultaneously in Japan and other countries.
- The device has the potential to address unmet medical needs in Japan. The target medical condition should be serious, life-threatening, or with persistent symptoms.
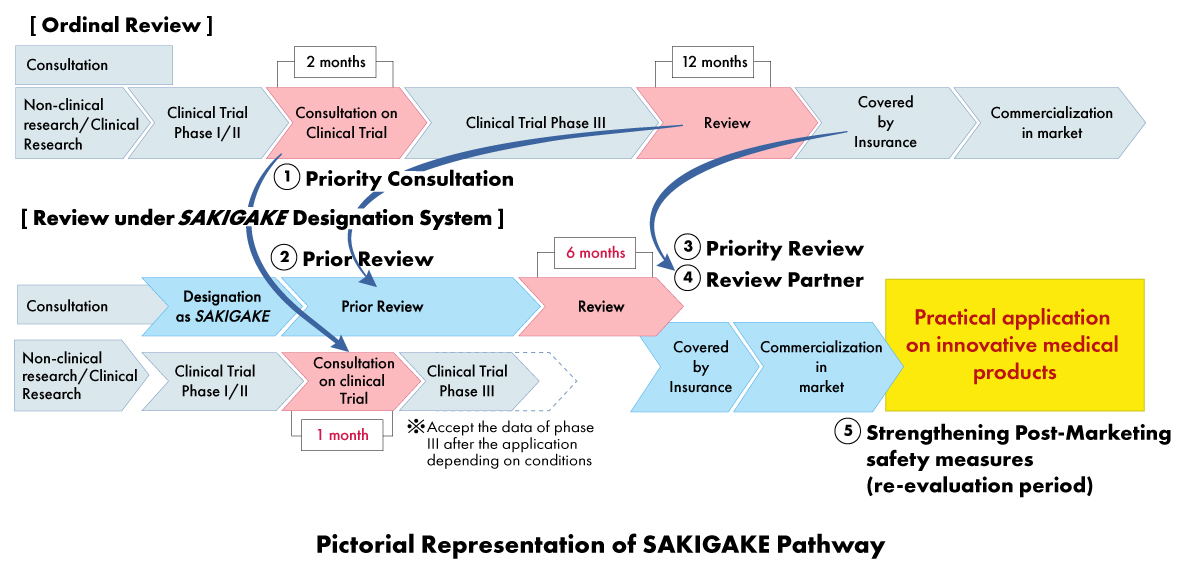
Under the SAKIGAKE strategy, medical device manufacturers can receive a range of benefits, including:
- Priority Consultation: Shortening the waiting time for clinical trial consultation to one (01) month.
- Priority Review: SAKIGAKE-designated products receive priority review, with the goal of completing the review process within six (06) months instead of 12 months.
- Effective Review before the Application: Encouraging consultation and accepting materials in English.
- Dedicated Review Partner: Each SAKIGAKE-designated product is assigned a dedicated review manager for the entire approval process.
- Strengthening Post-marketing Safety Measures: Extension of re-examination period and facilitating connection with scientific societies.
How to Apply?
- By Applicant: Applicant is submitting the application to the PMDA Evaluation and Licensing Division (ELD).
- By PMDA-ELD: ELD is approaching a potential applicant.
P.C- PMDA, Japan.
Overall, the SAKIGAKE system provides an accelerated pathway for the development and approval of innovative medical devices in Japan. It aims at improving patient outcomes and promoting innovation in the medical device industry. The manufacturer must consult the PMDA early in the development process to discuss Regulatory requirements and receive feedback on their product development plans.
To decode more about the medical device registration pathways in Japan, reach out to a regulatory expert now! Stay informed. Stay compliant.