
Artwork design is a crucial process in the supply of a pharmaceutical product, which primarily ensures patient safety while minimizing recall risk. In today’s highly regulated and digital-first pharmaceutical environment, Right First Time (RFT) artwork has become a key compliance and operational performance metric.
Artwork and labeling functions are an intrinsic part of the supply of a pharmaceutical product and are under constant pressure to deliver an increasing number of projects in a compressed timeframe. In the current business scenario, companies strive to differentiate themselves by reducing time to market, delivering a quick product mix, and rapidly adapting products to evolving market needs. Pharmaceutical and life sciences companies are governed by FDA validation, stringent quality norms, and GMP requirements. With increasing global inspections and digital audits, even minuscule artwork errors can be costly, damaging, and expose companies to significant compliance risks, including product recalls, health authority warnings, and fines.
The overall commercial success of a drug or pharmaceutical company is dependent on Right First Time artwork prior to entering new markets. The costs associated with pharmaceutical packaging account for up to 70% of the total cost of the finished product. It is also reported that 35–40% of product recalls are attributed to packaging and labeling errors and omissions. There have been a total of 455 product recall notices, out of which 51% were attributed to mislabeling and 13% due to faulty packaging, according to data collected over a six-month period by the US FDA. These statistics continue to reinforce the importance of robust artwork lifecycle management and packaging compliance strategies.
Globalization and Pharmaceutical Companies
Due to the expansion of global markets, pharmaceutical companies must contend with localization challenges, including language, cultural, and country-specific regulatory requirements. Emerging pharmaceutical markets pose additional challenges for companies seeking rapid market entry. Delivering Right First Time Artwork enables pharmaceutical companies to introduce new drugs efficiently while maintaining global labeling harmonization and regulatory consistency across regions.
Importance of Artwork Management: Supporting Product Launch and Patient Safety
Producing artwork for pharmaceuticals is a highly disciplined process requiring strict adherence to compliance, quality standards, and validation. Pharmaceutical products can only be sold if they are packaged correctly, and shipping can only occur if packaging text is absolutely accurate. If incorrect text is printed, a pharmaceutical company’s reputation and profitability can suffer severe setbacks. Artwork, as a function, is critical in supporting both product launches and patient safety. Today, artwork management systems (AMS) play a vital role in ensuring version control, audit readiness, and regulatory traceability.
Although the function does not offer a direct strategic competitive advantage, it demands strong GxP attention, similar to active ingredient efficacy, packing line clearance, and change control. Artwork design errors—even minimal—can result in catastrophic consequences and remain one of the leading causes of product recalls. Typically, artwork design involves coordinating information from multiple internal and external sources, increasing the risk of errors without standardized processes.
Product Recall: An Industry Estimate
- 35–40% of pharmaceutical product recalls are attributed to pack labeling errors and omissions
- 2–3 product recalls lead to significantly higher operational and remediation costs
- Lack of process standardization
- Staffing challenges leading to delays in artwork release
- Number of rework iterations ranging between 7 and 8 cycles
These challenges underscore the pressing need for centralized, technology-driven artwork governance models.
Approach: Process Improvement Complemented by Technology
Artwork processes are still largely managed through manual methods. Artwork management is a combination of project management, repository management, version control, regulatory labeling control, and the actual authoring process. Pharmaceutical companies must strategically develop streamlined processes to mitigate the risks associated with fragmented outsourcing models, which can increase compliance exposure and lead to recalls or regulatory penalties. Modern digital artwork management platforms, AI-enabled proofing, and automated compliance checks are now becoming industry best practices.
Robust Artwork Process
There has been increased industry momentum toward innovation in technology, stricter quality controls, and streamlined systems to create accurate, automated artwork workflows.
Key requirements include:
- Building an integrated, closed-loop artwork management process with continuous updates
- Establishing standard operating procedures (SOPs) and governance models
- Implementing appropriate artwork infrastructure and compliant software solutions
- Creating centralized artwork knowledge repositories to reduce rework
- Integrating artwork processes with R&D, regulatory affairs, and supply chain operations
These measures collectively enable scalable and sustainable Right First Time artwork capabilities.
Artwork Management Solution
Streamlining artwork management has become a key focus for the pharmaceutical industry, which invests heavily in packaging each year. Trusted, configurable artwork management solutions are replacing manual processes that are prone to defects and delays in layout creation and approvals. Automated solutions help close process gaps, enforce compliance, and accelerate timelines from design to print while improving transparency and accountability.
Collaboration Challenges
Artwork and labeling require collaboration across multiple internal and external stakeholders, including:
- Product Development
- Regulatory Affairs and Labeling Management
- Artwork Management and Vendor Collaboration
- Change Control
- Packaging and Manufacturing
Challenges arising from such collaboration include redundant processes, lack of end-to-end visibility, limited coordination, and inconsistent communication. Centralized artwork platforms and digital collaboration tools are increasingly being used to address these gaps.
Defined Artwork Management Process Assures Right-First-Time Artwork
A well-defined artwork process with clear control points ensures error-free execution:
Create source text → Define change → Produce artwork → Produce printer proof → Implement
Ensuring text accuracy through proofreading and verification before final printing remains critical.
Improvements to Artwork Capabilities: A Phased Approach
Step 1: In Control
- Compliant GxP processes
- Formal approvals at key control points
- Meeting critical artwork change milestones
Step 2: Efficient & Effective
- Meeting business performance requirements
- Optimal resource utilization
- Processes tuned for effective execution
Step 3: World Class
- Adoption of industry best practices
- External benchmarking
- Agile adaptation to changing regulatory and business models
Business Advantages of a Streamlined Artwork Function
A streamlined artwork function enhances quality by reducing recalls and launch delays resulting from artwork errors. It ensures branding consistency across designs, colors, and text, strengthening brand credibility. A standardized, audit-ready process across global markets reduces compliance penalties, improves staffing planning, enables artwork reuse across similar markets, and shortens launch timelines. Organizations also gain a better understanding of artwork change triggers and regulatory-driven business dynamics.
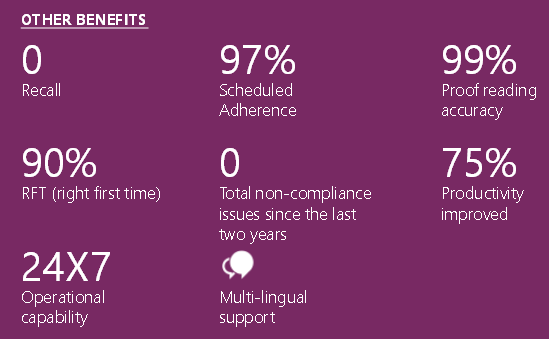
Delivering quality artwork is a complex endeavor involving multiple stakeholders, and packaging remains a significant compliance risk. Artwork management is critical to executing a pharmaceutical company’s business strategy. Right First Time Artwork can be achieved by assessing current processes against a comprehensive maturity model and conducting thorough gap analyses to identify opportunities for improvement across people, processes, and systems.









